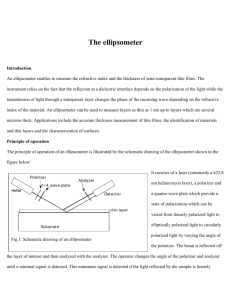
Electronic Supplementary Materials 2 Adhesion energy of air
advertisement

Electronic Supplementary Materials 2 Adhesion energy of air bubbles on a flat surface. As shown in the figure below, the energy balance within the area of interface (𝑆air ) between attachment (figure a) and detachment (figure b) is 𝛾S 𝑆air + 𝐸ad = (𝛾L + 𝛾SL )𝑆air (1) where 𝛾S , 𝛾L and 𝛾SL are surface energy per unit surface area (or surface tension) of the gas–solid, gas–liquid and solid–liquid interfaces, respectively. Therefore, the adhesion energy per unit area of interface (𝐸ad /𝑆air ) of the bubble on the flat surface is 𝐸ad /𝑆air = 𝛾L + 𝛾SL − 𝛾S , (2) where the right side is determined by the combination of liquid and solid, and 𝑆air is a function of the contact angle of bubble on the surface as [11] 𝑆air = π[4⁄(2 − 3 cos 𝜃air + cos 3 𝜃air )]2/3 𝑟02 sin2 𝜃air . (3) Here, 𝑟0 is the radius of the air bubble in water (figure b). The contact angle of air bubbles (𝜃air ) is determined by the wettability (hydrophilicity and hydrophobicity) of the surface. From the geometry in figure c, the balance between the surface tensions along the horizontal axis is 𝛾S = 𝛾SL + 𝛾L cos𝜃water . (4) From eq. (4) and 𝜃air + 𝜃water = 𝜋, we get the following equation: cos 𝜃air = cos(𝜋 − 𝜃water ) = − cos 𝜃water = (𝛾SL − 𝛾S )⁄𝛾L . (5) Therefore, the relationship between 𝐸ad /𝑆air and cos 𝜃air from eqs. (2) and (5) is 𝐸ad /𝑆air = 𝛾L (1 + cos𝜃air ). (6) Energy balance between the energies of adhesion and interface energies. (a) Attachment. (b) Detachment. (c) The balance among surface tensions to obtain Young’s relation. 𝛾S , 𝛾L and 𝛾SL are surface energy per unit surface area (or surface tension) of the gas– solid, gas–liquid and solid–liquid interfaces, respectively. 𝐸ad , adhesion energy of the bubble; 𝑟0 , radius of the air bubble; 𝑆air , area of interface; 𝜃air , contact angle of the bubble.