Chemistry Lesson Plan
advertisement

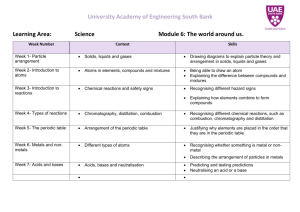
LEARNING—FOCUS STRATEGIES Lesson Planning Form Name: Sapp, Woods Class: 8th Science GPS: S8P1a: Distinguish between atoms Date(s): November 28 - December 9, and molecules 2011 S8P1b: Describe the difference between pure substance (elements and compounds) and mixtures S8P1f: Recognize that there are more than 100 elements and some have similar properties as shown on the Periodic Table of Elements Topic: Atoms, Elements, Periodic Table, Compounds and Mixtures Plan for the Concept, Topic, or Skill—Not for the Day Learning – Focus Time Essential Question(s): (What do students need to learn to be able to answer the Essential Question?) Use K-U-D organizer to categorize/combine into 2-4 Assessment Prompts (AP) Activating Thinking Strategies: (ex: kwl, word maps, wordsplash, etc…) How do I distinguish between atoms and molecules? How do I describe the difference between pure substance (elements and compounds) and mixtures? How do I recognize that there are more than 100 elements and some have similar properties as shown on the Periodic Table of Elements? AP#1 3-2-1 AP#2 Ticket out the door AP#3 Ticket out the door AP#4 summary AP#5 none AP#6 3-2-1 AP#7Ticket out the door AP#8:Ticket out the door AP#9:Best Test Questions AP#10: Test Read pg 323 Chapter 13 "The Atom" Key Vocabulary to Preview: 1. Atom 2. Nucleus 3. Proton 4. Electron 5. Neutron 6. Atomic number 7. Element 8. Molecules 9. Compound 10. Mixture 11. Pure substances 12. Metals 13. Non metals 14. Metalloid 15. Period 16. Group 17. Family Vocabulary: nice to know 18. Isotope 19. Mass number 20. Atomic mass 21. Solution 22. Suspension colloid Read pg 343 Chapter 14 "Elements/Periodic Table" Draw and label the parts of an atom: nucleus, proton, neutron, electron, electron cloud Label each part: atomic number, element name, symbol and mass number 3 Li Lithium 7 Read pg 363 Molecules and Compounds How do you make lemonade? CH4 What does each letter mean in a chemical formula? NaO, H2, HCl, H2O2 How many atoms are in each chemical formula? Teaching Strategies: AP’s are your embedded Summarizers throughout instructional period Graphic Organizers: Compare/Contrast: Compounds and Elements Compounds and Mixtures Tri-fold Elements, Compounds, and Mixtures Classifying: Elementsmetals, non-metals, and metalloids Cornell Note-Taking Format Word splash Frayer Model for vocabulary words Instruction for AP#1Notes for Atoms/ Elements Complete periodic table coloring activity Define vocabulary words for unit organizer AP#1 3-Sub atomic particles of an atom 2- parts of an atom 1-example of an element. Instruction for AP#2: Notes on Periodic Table Answer unit organizer questions AP#2: Where are the metalloids located on the periodic table? Instruction for AP#3 Review the periodic table with smart board activities, periodic tiles construct the periodic table in groups, complete periodic table puzzle AP#3 Where are the metals and nonmetals located on the periodic table? Instruction for AP#4:Safari MontageElements, compounds and mixtures Ap#4: Summary of video and video quiz Instruction for AP#5 Chapter 13 and 14 guided reading handouts AP#5 none(science planning) Instruction for AP#6 Notes on Compounds and Mixtures AP#6: 3-Examples of mixtures 2-examples of pure substances 1-difference between compound and elements Instruction for AP#7:Counting atoms and chemical formulas review, Virtual labs smart board activities; teacher demonstrations of solutions to identify solute and solvent in solutions. Mixtures, Solutions, and Compounds handouts Define vocabulary words for unit organizer AP#7: In a lemonade solution name the solute and solvent. Instruction for AP#8: Chemistry Review Sheet review chemical formulas and counting atoms, answer questions for unit organizer AP#8:What does a subscript in a chemical formula means? Instruction AP#9: Review for test Jeopardy Game or CPS units AP#9: Write 5 questions you may find on the test. Instruction for AP10: Test AP#10: Test Assignment and/or Assessment: Re-Teaching Focus and Strategy: (if necessary) SED Modifications: Materials and Resources Unit Organizer Quiz Review for Test using board game or CPS Final Assessment-Unit Test Reinforcement handout: Periodic Table, Elements and Compounds DH,CC, JP, EJ, DM, WE,-read aloud, repetition, paraphrase directions, extended time JA, MA-small groups( send to media center to test) ESOL Collaborative teacher-AV, AV, GP JH,PP, IG,-read aloud, repetition, paraphrase directions extended time CPO Physical Science Textbook Unit Organizer Glencoe pg 440 paragraph Science Holt chapter 10 Board Games Unit Test