C. Wilburn Lesson Plans Sept. 10-14, 2012 8th Grade Science Pre
advertisement
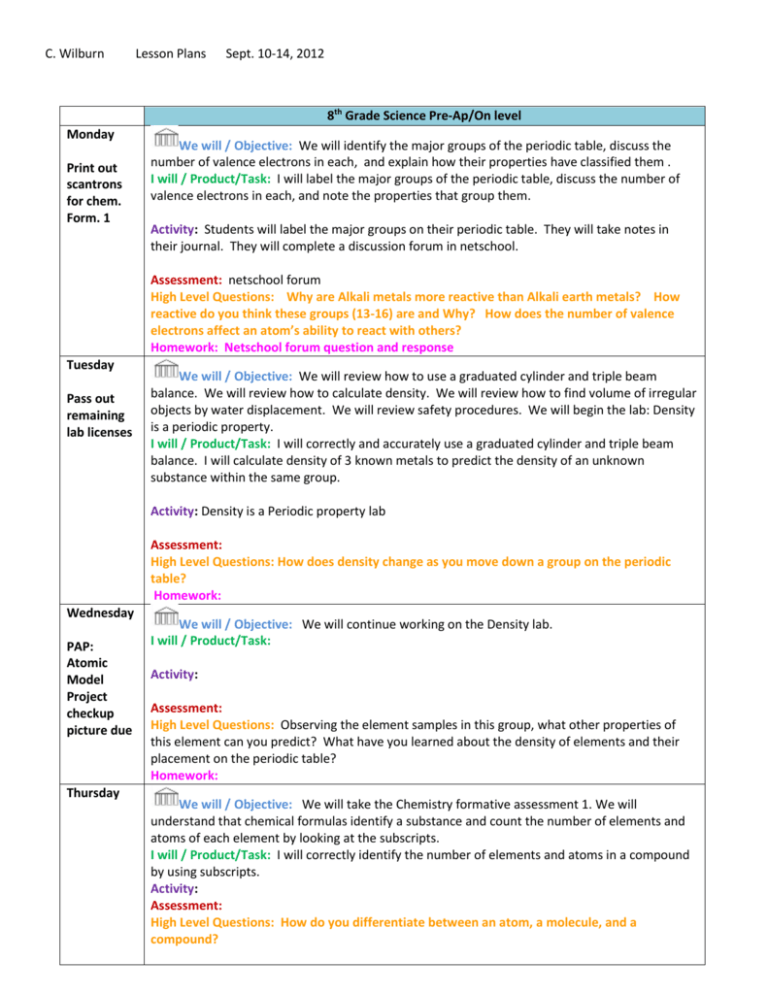
C. Wilburn Lesson Plans Sept. 10-14, 2012 8th Grade Science Pre-Ap/On level Monday Print out scantrons for chem. Form. 1 We will / Objective: We will identify the major groups of the periodic table, discuss the number of valence electrons in each, and explain how their properties have classified them . I will / Product/Task: I will label the major groups of the periodic table, discuss the number of valence electrons in each, and note the properties that group them. Activity: Students will label the major groups on their periodic table. They will take notes in their journal. They will complete a discussion forum in netschool. Assessment: netschool forum High Level Questions: Why are Alkali metals more reactive than Alkali earth metals? How reactive do you think these groups (13-16) are and Why? How does the number of valence electrons affect an atom’s ability to react with others? Homework: Netschool forum question and response Tuesday Pass out remaining lab licenses We will / Objective: We will review how to use a graduated cylinder and triple beam balance. We will review how to calculate density. We will review how to find volume of irregular objects by water displacement. We will review safety procedures. We will begin the lab: Density is a periodic property. I will / Product/Task: I will correctly and accurately use a graduated cylinder and triple beam balance. I will calculate density of 3 known metals to predict the density of an unknown substance within the same group. Activity: Density is a Periodic property lab Assessment: High Level Questions: How does density change as you move down a group on the periodic table? Homework: Wednesday PAP: Atomic Model Project checkup picture due Thursday We will / Objective: We will continue working on the Density lab. I will / Product/Task: Activity: Assessment: High Level Questions: Observing the element samples in this group, what other properties of this element can you predict? What have you learned about the density of elements and their placement on the periodic table? Homework: We will / Objective: We will take the Chemistry formative assessment 1. We will understand that chemical formulas identify a substance and count the number of elements and atoms of each element by looking at the subscripts. I will / Product/Task: I will correctly identify the number of elements and atoms in a compound by using subscripts. Activity: Assessment: High Level Questions: How do you differentiate between an atom, a molecule, and a compound? Homework: Friday We will / Objective: We will complete the activity Building atom models and identify them as organic or inorganic and write the correct chemical formula for each. I will / Product/Task: I will create Bohr models to represent atoms that combine and form compounds. I will identify them as organic or inorganic. I will correctly write the chemical formulas including subscripts. Activity: Students will create several Bohr models of molecules and compounds in groups. Students will answer questions in their journals. Assessment: High Level Questions: What happens when an atom gains or loses an electron? Homework: Key Understand ings Key Vocabulary Upcoming Due Dates TEKS Element, compounds, formulas, molecule, organic, inorganic Pre-AP: Atomic Model Project Checkup Wednesday 8.5D Recognize (to identify from knowledge of appearance or characteristics) that chemical formulas are used to identify substances and determine (to find out or come to a decision about by investigation, reasoning, or calculation) the number of atoms of each element in chemical formulas containing subscripts. 8.3B Use models to represent aspects of the natural world such as an atom, a molecule, space, or a geologic feature. 7.6A Identify that organic compounds contain C and other elements such as H, O , P, N, or S. 6.5C Differentiate between elements and compounds on the most basic level.











