Uncertainty & Errors in Measurement

Uncertainty & Errors in
Measurement
Waterfall by M.C. Escher
Keywords
Uncertainty
Precision
Accuracy
Systematic errors
Random errors
Repeatable
Reproducible
Outliers
Measurements = Errors
Measurements are done directly by humans or with the help of
Humans are behind the development of instruments, thus there will always be associated with all instrumentation, no matter how precise that instrument is.
Uncertainty
When a physical quantity is taken, the uncertainty should be stated.
Example
If the balance is accurate to +/- 0.001g, the measurement is 45.310g
If the balance is accurate to +/- 0.01g, the measurement is 45.31g
Exercise
A reward is given for a missing diamond, which has a reported mass of 9.92 +/-
0.05g. You find a diamond and measure its mass as 10.1 +/- 0.2g. Could this be the missing diamond?
Significant Figures
(1)
(2)
____ significant figures in 62cm 3
____ significant figures in 100.00 g.
The 0s are significant in (2) What is the uncertainty range?
Measurements Sig. Fig.
1000s Unspecified
1 x 10 3 s
1.0 x 10 3 s
1.00 x 10 3 s
1.000 x 10 3 s
1
2
3
4
Measurements
0.45 mol dm -3
4.5 x 10 -1 s mol dm -3
4.50 x 10 -1 s mol dm -3
4.500 x 10 -1 s mol dm -3 4
4.5000 x 10 -1 s mol dm -3 5
2
3
Sig. Fig.
2
Random (Precision) Errors
An error that can based on
individual interpretation.
Often, the error is the result of mistakes or errors.
Random error is not ______ and can fluctuate
up or down. The smaller your random error is, the greater your ___________ is.
Random Errors are caused by
The readability of the measuring instrument.
The effects of changes in the surroundings such as temperature variations and air currents.
Insufficient data.
The observer misinterpreting the reading.
Minimizing Random Errors
By repeating measurements.
If the same person duplicates the experiment with the same results, the results are repeatable.
If several persons duplicate the results, they are reproducible.
10 readings of room temperature
19.9 , 20.2 , 20.0, 20.0, 20.1, 19.9, 20.3, 19.9, 20.2, 22.3
(a) What is the mean temperature?
The temperature is reported as as it has a range of
Read example in the notes .
Systematic Errors
An error that has a fixed margin, thus producing a result that differs from the true value by a fixed amount.
These errors occur as a result of poor experimental design or procedure.
They cannot be reduced by repeating the experiment.
10 readings of room temperature
20.0 , 20.3 , 20.1, 20.1, 20.2, 20.0, 20.4, 20.0, 20.3
19.9 , 20.2 , 20.0, 20.0, 20.1, 19.9, 20.3, 19.9, 20.2
All the values are ____________.
(a) What is the mean temperature?
The temperature is reported as
Examples of Systematic Errors
Measuring the volume of water from the top of the meniscus rather than the bottom will lead to volumes which are too ________.
Heat losses in an exothermic reaction will lead to ______ temperature changes.
Overshooting the volume of a liquid delivered in a titration will lead to volumes which are too ______ .
Minimizing Systematic Errors
Control the variables in your lab.
Design a “perfect” procedure ( not ever realistic)
Errors
Systematic errors
Apparatus
Random errors
Equal chance of reading being high or low from
1 measurement to the next
Way in which readings are taken
How trustworthy is your reading?
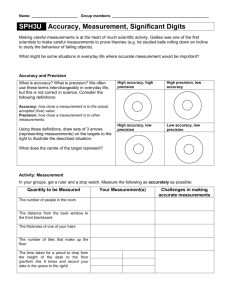
Accuracy
• How close to the accepted(true) value your reading is.
Precision
• The reproducibility of your reading
• Reproducibility does
not guarantee
accuracy. It could simply mean you have a very determinate
systematic error.
If all the temperature reading is 20 0 C but the true reading is 19 0 C .
This gives us a precise but inaccurate reading.
If you have consistently obtained a reading of 20 0 C in five trials. This could mean that your thermometer has a large systematic error.
systematic error accuracy random error precision
systematic error accuracy random error precision
Exercise
Putting it together
Example
The accurate pH for pure water is 7.00 at 25 0 C.
Scenario I
You consistently obtain a pH reading of
6.45 +/- 0.05
Accuracy:
Precision:
Scenario II
You consistently obtain a pH reading of
8 +/-2
Accuracy:
Precision:
Calculations involving addition & subtraction
When adding and subtracting, the final result should be reported to the same number of
decimal places as the least no. of decimal places.
Example:
(a) 35.52 + 10.3 (b) 3.56 – 0.021
Calculations involving multiplication
& division
When adding and subtracting, the final result should be reported to the same number of
significant figures as the least no. of significant figures .
Example:
(a) 6.26 x 5.8 (b)
5.27
12
Example
When the temperature of 0.125kg of water is increased by 7.2
0 C. Find the heat required.
Heat required
= mass of water x specific heat capacity x temperature rise
= 0.125 kg x 4.18 kJ kg -1 0 C -1 x 7.2
0 C
=
Since the temperature recorded only has 2 sig fig, the answer should be written as ____________
Multiple math operations
Example:
5.254 + 0.0016
2.231×10
-3
34.6
Uncertainties in calculated results
These uncertainties may be estimated by
from the smallest division from a scale from the last significant figure in a digital measurement from data provided by the manufacturer
Absolute & Percentage Uncertainty
Consider measuring 25.0cm
3 with a pipette that measures to +/- 0.1 cm 3 .
We write
Absolute Uncertainty
cm
3
Percentage Uncertainty
0.1
25.0
100%
0.4%
The uncertainties are themselves approximate and are generally not reported to more than
1 significant fgure.
Percentage Uncertainty &
Percentage Error
Percentage uncertainty = absolute uncertainty
100% measured value
Percentage error = accepted value-experimental value
100% accepted value
When adding or subtracting measurement , add the absolute uncertainties
Example
Initial temperature = 34.50
0 C
Final temperature = 45.21
0 C
0
0
Change in temperature, ΔH
When multiplying or dividing measurement, add the percentage uncertainties
Example volume = 14.1 cm 3
What is the density?
0.05cm
3
Example
Calculate the following:
(a)
5.2 0.1
m
m
(b) m
m
Example:
initial volume from the final volume. The volume delivered is
Final vol = 38.46
Initial vol = 12.15
What is total volume delivered?
Example
The concentration of a solution of hydrochloric acid = moldm and the volume = cm 3 .
10.0 0.1
Calculate the number of moles and give the absolute uncertainty.
When multiplying or dividing by a pure number, multiply or divide the uncertainty by that number
Example
Powers :
When raising to the nth power, multiply the
percentage uncertainty by n.
When extracting the nth root, divide the
percentage uncertainty by n.
Example
4.3 ± 0.5cm
3
Averaging : repeated measurements can lead to an average value for a calculated quantity.
Example
Average ΔH
=[+100kJmol -1 ( 10%)+110kJmol -1 ( 10%)+
108kJmol -1 ( 10%)] 3
= 106kJmol -1 ( 10%)]
Calculations
Add & Subtract Multiply & Divide
No. of decimal places
No. of significant figures
No. with the fewest sig fig used determines the sig fig to be used for the answer.
Factory made thermometers
Assume that the liquid in the thermometer is calibrated by taking the melting point at
0 0 C and boiling point at 100 0 C (1.01kPa).
If the factory made a mistake, the reading will be biased.
Instruments have measuring scale identified and also the tolerance .
Manufacturers claim that the thermometer reads from -10 0 C to 110 0 C with uncertainty +/- 0.2
0 C.
Upon trust, we can reasonably state the room temperature is
20.1
0 C +/- 0.2
0 C.
Graphical Technique
y-axis : values of dependent variable x-axis : values of independent variables
Plotting Graphs
Give the graph a title.
Label the axes with both quantities and units.
Use sensible linear scales – no uneven jumps.
Plot all the points correctly.
A line of best fit should be drawn clearly. It does not have to pass all the points but should show the general trend.
Identify the points which do not agree with the general trend.
Line of Best Equation
74,0
Change in volume of a fixed gas heated at a constant pressure
Temperature ( 0 C) Volume of Gas (cm 3 )
20.0
60.0
30.0
40.0
63.0
64.0
50.0
60.0
70.0
67.0
68.0
72.0
72,0
70,0
68,0
66,0
64,0
62,0
60,0
58,0
0,0 10,0 20,0 30,0 40,0 temperature ( 0 C)
50,0 60,0 70,0 80,0
Graphs can be useful to us in predicting values.
Interpolation – determining an unknown value within the limits of the values already measured.
Extrapolation – requires extending the graph to determine an unknown value that lies outside the range of the values measured.