Experiment 3 - Chemical and Physical Properties
advertisement

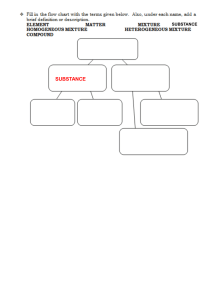
Experiment 3 – Chemical and Physical Properties Name __________________ Lab Section __________________ Experiment 3 – Chemical and Physical Properties of Materials Introduction Matter, the “stuff” that makes up everything we see, can be classified in several manners. It can be found as a pure substance, an element or compound, or a mixture. Each of these will, in turn, have both physical and chemical properties. To investigate these properties of matter, a few definitions must be made. Pure substances can either be elements or compounds. Elements are anything found on the periodic table. As long as the matter is comprised only of that element, it is a pure substance. A chunk of copper, oxygen gas, a lead weight, and a diamond (pure carbon) are prime examples of a pure substance that is in an elemental form. A compound is also a pure substance made up of more than one element, as long as there are no impurities. Pure water, sodium chloride, calcium carbonate, and aluminum oxide, are examples of a pure substance in the form of a compound. Mixtures occur when two or more elements or compounds are mixed together. There are two types of mixtures, homogeneous and heterogeneous. Homogeneous mixtures are when the properties of the solution are the same throughout. Examples of this would be salt water, air, vanilla ice cream, and metal alloys like stainless steel. Heterogeneous mixtures are when the properties can change, depending on where you are looking in the solution. Examples of this would be and oil/water mixture, rocky road ice cream, and raw metal ores (metal mixed in with dirt and such). Each of these classifications of matter has both chemical and physical properties. A physical property is one that can be observed without altering the substance. That is to say, it remains unchanged after the observation. Examples of this include, but are not limited to, color, phase changes (boiling, melting, and freezing points), solubility, miscibility, hardness, and density. Each of these is observable without creating a new substance. A chemical property is essentially whether or not the substance will react with another substance. For instance, iron reacting with oxygen to form rust, and the fact that argon does not react with anything, are both chemical properties. Chemical properties are observed in a chemical reaction and a new substance is formed. A physical property is determined when a physical change takes place. A physical change is one in which the substance undergoes a change, but its composition remains the same. Examples of this are a temporary color change (as in the heating of metal), breaking something in half to determine its strength, or dissolving a substance in water. A chemical change is observed in a chemical reaction. There are four ways to know if a chemical change has occurred. These evidences are: permanent color change, gas being released, formation of a solid (precipitate, abbreviated as PPT), and heat released/absorbed. Examples of these would be iron rusting, antacid tablets fizzing in water, a solution turning a milky color when mixed, and a solution heating up when mixed. Experiment 3 – Chemical and Physical Properties Name __________________ Lab Section __________________ Prodecure Physical properties – Observations of Elements Using the given samples of elements, describe their physical characteristics, determine their symbol, and indicate whether or not they are a metal, semi metal, or nonmetal. Physical properties – Solubility of a Solid in Water – Soluble or Insoluble Half fill two small test tubes with water. Add a few crystals of copper (II) sulfate, CuSO4, into one test tube, and a small chunk of calcium carbonate, CaCO3 to the other. Gently shake the test tubes and note whether or not the solids dissolve. Note whether or not the solids are soluble or insoluble. Physical properties – Solubility of a Liquid in a Liquid – Miscible or Immiscible Add 20-30 drops, ~1 mL, of water into two test tubes. Add 20-30 drops of ethanol to one test tube and 20-30 drops of hexane to the other. Note whether or not the solutions mix, and indicate whether they are miscible or immiscible. Chemical properties – Reactions of Elements (Instructor Demo) Observe a copper wire before and after being heated with a Bunsen burner. Indicate whether the wire underwent a chemical or physical change. Observe iodine crystals before and after being heated. Indicate whether the iodine underwent a chemical or physical change. Chemical properties – Reactions of Compounds Add 20 drops of sodium carbonate, Na2CO3, and 20 drops of sodium sulfate, Na2SO4, into separate, small test tubes. Add hydrochloric acid, HCl, to each the test tube and note any changes that might occur. Indicate if the addition of hydrochloric acid resulted in a chemical or physical change. Add 20 drops of calcium nitrate, Ca(NO3)2, and 20 drops of copper (II) nitrate, Cu(NO3)2, into separate, small test tubes. Add 20 drops of ammonium hydroxide, NH4OH, to each test tube and note any changes that might occur. Indicate if the addition of ammonium hydroxide resulted in a chemical or physical change. Experiment 3 – Chemical and Physical Properties Name __________________ Lab Section __________________ Boiling Point Measurement Boiling Point – Methanol Refer to Figure 3.1 for the setup of the boiling point apparatus. Fill a 400 mL beaker ¾ full with water, add a boiling chip to the beaker, and support it on a ring stand with an iron ring and wire gauze. Add between 1-2 mL of methanol into a large test tube and put a boiling chip into the methanol. Place a thermometer into a split rubber stopper and suspend the end of the thermometer approximately 1 cm above the methanol. Use a test tube clamp to hold the test tube to the ring stand. Bring the water to a boil using the Bunsen burner and shut off the burner. Lower the methanol into the beaker. The alcohol will begin to boil and liquid will begin dripping from the bottom of the thermometer. When this is observed, read the thermometer. When the temperature no longer continues to rise, record the temperature. Be careful not to allow the methanol to completely boil off. Safety Note: Methanol is very flammable! It is imperative that the Bunsen burner be shut off before the methanol is introduced to the beaker to avoid possible vapor ignition. Keep all flames far away from the methanol! Boiling Point – Unknown Liquid Obtain an unknown liquid from your instructor and record its number. Repeat the instructions above using your unknown liquid. Figure 3.1 Experiment 3 – Chemical and Physical Properties Name __________________ Lab Section __________________ Prelaboratory Questions What are the four ways you know a chemical reaction has taken place? Give your own example of: Homogeneous mixture Heterogeneous mixture Element Compound Physical Change Chemical Change Is each of the following a chemical or physical property: Iron rusts ____________ Rust is red in color ____________ Iron is malleable ____________ Iron’s density is 5.8𝑐𝑚3 𝑔 ____________ Is each of the following a chemical or physical change: Gasoline burns in air ____________ Ice melts ____________ Salt dissolves in water____________ Milk sours ____________ Experiment 3 – Chemical and Physical Properties Name __________________ Lab Section __________________ Data Table Physical Properties – Observations of Elements Element Symbol Physical Characteristics Metal/Semi/Nonmetal Oxygen Silicon Argon Magnesium Aluminum Iron Sulfur Phosphorous Mercury Cobalt Tin Sodium Arsenic Physical Properties – Solubility of a Solid in Water Observation Soluble or Insoluble? Copper sulfate in water ________________ ___________________ Calcium carbonate in water ________________ ___________________ Physical Properties – Miscibility of a Liquid in Water Observation Miscible or Immiscible? Ethyl alcohol in water ________________ ___________________ Hexane in water ________________ ___________________ Experiment 3 – Chemical and Physical Properties Name __________________ Lab Section __________________ Chemical Properties – Reactions of Elements (Instructor Demo) Observation Physical or Chemical Change Copper wire and heat ____________________ _______________ Iodine and heat ____________________ _______________ Chemical Properties – Reactions of Compounds Potassium hydrogen carbonate And heat ____________________ ________________ Ammonium hydrogen carbonate And heat ____________________ ________________ Chemical Properties – Reactions of Solutions Sodium carbonate and Hydrochloric acid ____________________ ________________ Sodium sulfate and Hydrochloric acid ____________________ ________________ Calcium nitrate and Ammonium hydroxide ____________________ ________________ Copper (II) nitrate and Ammonium hydroxide ____________________ ________________ Boiling Point Measurement Boiling Point – Methanol ________ °C Boiling Point – Unknown #_______ ________ °C Experiment 3 – Chemical and Physical Properties Name __________________ Lab Section __________________ Postlaboratory Questions 1) Indicate whether the following properties are representative of a metal, semi metal, or nonmetal: Left hand side of the Periodic Table _____________ Brittle _____________ Can behave like a metal or nonmetal _____________ Malleable _____________ High melting point _____________ Reacts with nonmetals _____________ Reacts with metals and nonmetals _____________ 2) Classify each of the following as an element, compound, homogeneous mixture, or heterogeneous mixture. Mercury metal, Hg __________ Amalgum fillings (silver in mercury) __________ mercury (II) chloride, HgCl2 __________ mercury in sand __________ 3) Indicate whether each of the following is a chemical or physical property: Color of the material __________ Hardness __________ Boiling point __________ Reacts with oxygen __________ 4) Indicate whether each of the following is a chemical or physical change: Tomato ripens __________ Frying an egg __________ Water boils when heated __________ Chopping wood in half __________