Ions and Ionic Compounds
advertisement
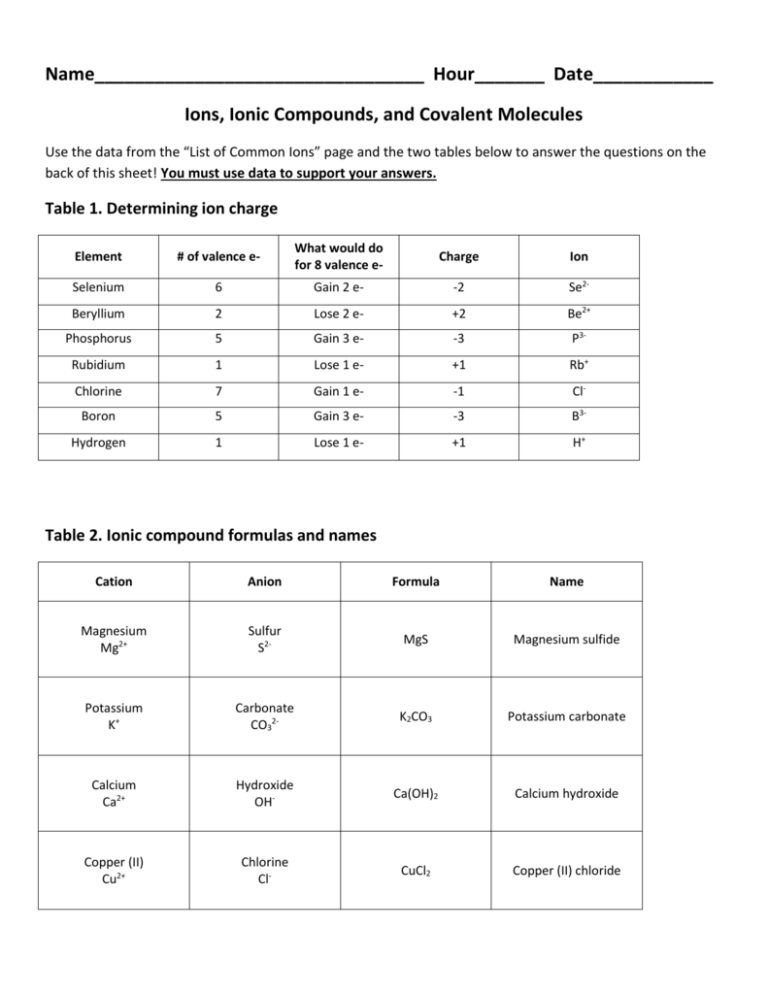
Name_________________________________ Hour_______ Date____________ Ions, Ionic Compounds, and Covalent Molecules Use the data from the “List of Common Ions” page and the two tables below to answer the questions on the back of this sheet! You must use data to support your answers. Table 1. Determining ion charge Element # of valence e- What would do for 8 valence e- Charge Ion Selenium 6 Gain 2 e- -2 Se2- Beryllium 2 Lose 2 e- +2 Be2+ Phosphorus 5 Gain 3 e- -3 P3- Rubidium 1 Lose 1 e- +1 Rb+ Chlorine 7 Gain 1 e- -1 Cl- Boron 5 Gain 3 e- -3 B3- Hydrogen 1 Lose 1 e- +1 H+ Table 2. Ionic compound formulas and names Cation Anion Formula Name Magnesium Mg2+ Sulfur S2- MgS Magnesium sulfide Potassium K+ Carbonate CO32- K2CO3 Potassium carbonate Calcium Ca2+ Hydroxide OH- Ca(OH)2 Calcium hydroxide Copper (II) Cu2+ Chlorine Cl- CuCl2 Copper (II) chloride Data analysis and model development: Ions and IONIC COMPOUNDS When answering all of these questions, you MUST use data from the tables provided. The following is an acceptable answer to the first question: monoatomic ions (such as Na+) are composed of an atom from one element whereas polyatomic ions (such as CO32-) are composed of more than one atom from different elements. Na+ is composed of one sodium atom whereas CO32- contains a carbon and oxygen atom. Na+ and CO32-are similar in that both contain an uneven number of electrons and protons, resulting in a positive or negative charge. Using list of common ions 1. What is similar and different between monoatomic and polyatomic ions? 2. What is different between cations and anions? 3. How can you determine the charge of a Type A element’s ion? 4. How are transition metals different than other elements in the periodic table in the ions they form? 5. What is different about naming a monoatomic and polyatomic ion? Using Table 1 and Table 2 from the front page 6. What is the relationship between an element’s number of valence electrons and the charge developed when it is an ion? 7. Why do metals lose electrons when forming an ion and nonmetals gain electrons to form an ion? 8. What patterns can you see in determining the formula of an ionic compound given the ions that compose it? 9. How do you name an ionic compound? 10. What are ionic compounds? 11. Write down 5 rules to determining ions, writing ionic compounds, and naming ionic compounds. Data analysis and model development: Ions and COVALENT MOLECULES When answering all of these questions, you MUST use data from the tables provided. Lewis Structure: A Lewis structure is a way of showing the number of valence electrons each element’s atoms have. Look at the table below to identify trends. Octet Rule: Elements want to gain or lose electrons to have the same electron configuration as the nearest noble gas. Thus, they want to have 8 valence electrons. Figure 1. Trends in Lewis Structures Figure 2. Listings of Electronegativities of Each Element Figure 3. Determination of Bond Type Based on Electronegativity Difference Table 1. Covalent molecule formulas and names Element #1 Number of Single Covalent bonds Element #2 Formula Name Oxygen Oxygen Number of Single Covalent Bonds 2 2 Carbon Hydrogen 4 1 CO2 H2O 1 Chlorine 1 HCl 4 Hydrogen 1 CH4 Carbon dioxide Dihydrogen oxide (water) Hydrogen chloride (hydrochloric acid) Carbon tertahydride (methane) Hydrogen Carbon Using Figure 1 1. What does each dot around the element symbol for a Lewis structure represent? 2. What do you notice about all of the elements in the same family (column)? 3. Looking at the halogen column, how many electrons does a halogen want to take or share in order to fulfill the octet rule? How does the Lewis structure for all of the halogens show this? 4. An element can gain or lose electrons by forming bonds. When an element shares two valence electrons (one of its own and one from another element) a single covalent bond is formed. How many single covalent bonds would each halogen form? 5. How many single covalent bonds would all elements in the oxygen column form? Using Figure 2 1. Metals give away electrons to non-metals when they form bonds; this bond is called an ionic bond. What do you notice about the electronegativities of metals and non-metals? 2. Why isn’t the noble gas column on the table of Figure 2? Using Figure 3 1. Using the difference in electronegativites found in Figure 3, explain why covalent bonds only occur between nonmetals. 2. Using Figure 3, make a prediction about the difference between nonpolar covalent bonds and polar covalent bonds. 3. What is the difference between a covalent bond and an ionic bond? Using Table 1 1. What patterns can you identify between the number of covalent bonds each element in a covalent molecule can make and the formula? 2. What patterns did you notice in the naming of covalent molecules?






