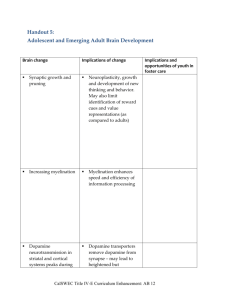
Chapter 1. General Introduction
advertisement

Neurocognitive Risk and Protective Factors in Addictive Disorders Dana G. Smith King’s College A dissertation submitted for the degree of Doctor of Philosophy at the University of Cambridge Declaration This dissertation is the result of my own work and includes nothing that is the outcome of work done in collaboration, except where specifically indicated in the text. This dissertation does not exceed the word limit specified by the School of Biological Sciences and has not been submitted in whole or in part for any other degree or qualification at any other university. The research reported in this dissertation has been presented in the following publications: Smith DG, Jones PS, Bullmore ET, Robbins TW, & Ersche KD. (2013). Enhanced orbitofrontal cortex function and lack of attentional bias to cocaine cues in recreational stimulant users. Biological Psychiatry. Published online. Smith DG, Jones PS, Williams GB, Bullmore ET, Robbins TW, & Ersche KD. (2013). Overlapping decline in orbitofrontal gray matter volume related to cocaine use and body mass index. Addiction Biology. Published online. Smith DG, Jones PS, Bullmore ET, Robbins TW, & Ersche KD. (2013). Cognitive control dysfunction and abnormal frontal cortex activation in stimulant drug users and their siblings. Translational Psychiatry. 3: e257. Ersche KD, Jones PS, Williams GB, Smith DG, Bullmore ET, & Robbins TW. (2013). Distinctive personality traits and neural correlates associated with stimulant drug use versus familial risk of stimulant dependence. Biological Psychiatry. 74: 137144. 3 Smith DG & Robbins TW. (2012). The neurobiological underpinnings of obesity and binge eating: A rationale for adopting the food addiction model. Biological Psychiatry. 73: 804-810. Robbins TW, Gillan CM, Smith DG, de Wit S, & Ersche KD. (2012). Neurocognitive endophenotypes of impulsivity and compulsivity: Towards dimensional psychiatry. Trends in Cognitive Science. 16: 81-91. 4 Acknowledgments Thank you, first and foremost, to Trevor Robbins and Karen Ersche for giving me this opportunity and for all of their guidance, knowledge and support throughout the PhD. I have learned so much from you both. Thank you to Annemieke Apergis-Schoute, Ed Bullmore, Gonzalo Urcelay, Mercedes Arroyo, Naresh Subramaniam, Sanja Abbot, Sharon Morein-Zamir and the other members of the BCNI for their patience and advice. Special thanks to Simon Jones for his invaluable help with fMRI analyses. Thank you to Abigail Turton, Claire Whitelock, Rania Christoforou and Heatha Agyepong for their assistance with data collection and organization during the ICCAM project. Thank you to Claire Gillan, Yasemin Yazar and Jane Garrison for sharing their time, space and chocolate with me. Thank you to all the participants who took part in these studies for sharing their stories. Thank you to the Cambridge Overseas Trust for providing the funding for my PhD. Thank you to the King’s College Graduate community for providing much-needed distraction, commiseration, humour and friendship throughout these three years. Thank you especially to Ruth Watkinson and Sam Greenbury Thank you to my parents, without whose love and support I could not have done this. 5 6 Abstract Cognitive impairments and changes in the structure and function of related brain regions, namely the prefrontal cortex and striatum, have long been implicated in drug addiction. However, it is unknown whether these abnormalities predate substance abuse, potentially serving as risk factors for dependence, or if they are the consequence of protracted use. To address this question, endophenotype research using stimulant-dependent individuals’ biological siblings has been used to investigate traits implicated in the pathology of addiction. Impairments present in both groups suggest an underlying risk-state for dependence, while additional abnormalities present only in stimulant-dependent individuals reflect potential effects of the drugs themselves. Contrastingly, there are also individuals who use stimulant drugs in a controlled manner without developing dependence. These ‘recreational users’ may lack the underlying traits that comprise a greater risk for dependence, or they might maintain additional protective factors against the development of addiction. Experiments in the first half of this dissertation used functional magnetic resonance imaging to investigate neurocognitive similarities and differences between dependent stimulant users, their non-dependent siblings, recreational users of cocaine, and unrelated healthy control volunteers. In Chapter 2, performance on a colour-word Stroop task was impaired in both stimulant-dependent individuals and their siblings, suggesting an endophenotype of cognitive inefficiency. However, neural activity significantly differed between the groups, indicating additional changes specific to the use of stimulant drugs. In Chapter 3, dependent users showed significant attentional bias to salient stimuli on a cocaine-word Stroop task, with a concurrent increase in prefrontal activation. Conversely, recreational users showed resilience in the face of cocaine cues and a decrease in arousal. Finally, Chapter 4 explored differences in reward sensitivity to 7 both generic and drug-specific reinforcers, comparing the effects of personal and family history of stimulant exposure on a monetary incentive delay task. It is also under debate whether the neurocognitive differences seen in stimulantdependent individuals are unique to substance abuse, or if parallel changes in behaviour and neurobiology are present in similar addiction-spectrum disorders, such as binge eating leading to obesity. In Chapter 5, stimulant-dependent and obese individuals with binge-eating behaviours showed differences in their substance-specific and general reward responsivity on a novel reward-valuation task. However, in Chapter 6 a similar decline in orbitofrontal cortex grey matter volume in relation to both years of stimulant use and body mass index was identified, implicating an overlap in this area between both conditions. These findings are integrated in Chapter 7, discussing the neurocognitive risk and protective factors that underlie an individual’s vulnerability for addiction, not only to stimulant drugs, but also potentially for other addictive behaviours. 8 Table of Contents Chapter 1. General Introduction 1. Definition of Addiction .............................................................................................. 13 2. Striatal Dopamine System and Bottom-Up Motivation ............................................. 14 2.1 Incentive Salience ................................................................................................. 15 2.2 Transition from Goal-Directed to Habitual Drug Behaviour ............................... 16 3. Dopamine System Abnormalities in Stimulant Dependence ..................................... 17 3.1 Genetic Variants Implicated in Addiction ............................................................ 19 4. Impulsivity ................................................................................................................. 19 5. Prefrontal Cortex and Top-Down Control ................................................................. 22 5.1 Cognitive Impairment ........................................................................................... 24 6. Orbitofrontal Cortex ................................................................................................... 25 6.1 OFC Abnormalities in Stimulant Dependence ..................................................... 26 6.2 Reward Valuation ................................................................................................. 26 6.3 Attentional Bias .................................................................................................... 28 6.4 Compulsive Behaviour ......................................................................................... 29 7. Reward System Abnormalities ................................................................................... 30 8. Endophenotype Research ........................................................................................... 32 9. Recreational Stimulant Use ........................................................................................ 34 10. Food Addiction ......................................................................................................... 36 11. Aims and Objectives ................................................................................................ 38 Chapter 2. Cognitive Control Dysfunction and Abnormal Frontal Cortex Activation in Stimulant Drug Users and their Biological Siblings 1. Introduction ................................................................................................................ 41 2. Materials and Methods ............................................................................................... 43 2.1 Participants ........................................................................................................... 43 2.2 Measures ............................................................................................................... 46 2.3 Procedure .............................................................................................................. 46 2.4 Imaging Acquisition ............................................................................................. 47 2.5 Data Analysis ........................................................................................................ 47 3. Results ........................................................................................................................ 49 9 3.1 Stroop Performance .............................................................................................. 50 3.2 Neuroimaging Analysis ........................................................................................ 51 3.3 Regression of Stroop Performance to Neuroimaging Findings ............................ 56 3.4 Relationship of Structural Changes to Stroop Performance ................................. 56 3.5 Effects of Drug Use History on Performance ....................................................... 58 4. Discussion .................................................................................................................. 58 Chapter 3. Enhanced Orbitofrontal Cortex Function and Lack of Attentional Bias to Cocaine Cues in Recreational Stimulant Users 1. Introduction ................................................................................................................ 63 2. Materials and Methods ............................................................................................... 66 2.1 Participants ........................................................................................................... 66 2.2 Behavioural Measures .......................................................................................... 68 2.3 Behavioural Analysis ............................................................................................ 69 2.4 Imaging Analysis .................................................................................................. 69 3. Results ........................................................................................................................ 71 3.1 Behavioural Performance ..................................................................................... 71 3.2 Functional Imaging ............................................................................................... 72 4. Discussion .................................................................................................................. 77 Chapter 4. A Comparison of Endophenotype and Stimulant Exposure Effects in Reward Sensitivity 1. Introduction ................................................................................................................ 82 2. Materials and Methods ............................................................................................... 85 2.1 Participants ........................................................................................................... 85 2.2 Behavioural Task .................................................................................................. 85 2.3 Behavioural Analysis ............................................................................................ 88 2.4 Imaging Analysis .................................................................................................. 88 3. Results ........................................................................................................................ 90 3.1. Demographic ....................................................................................................... 90 3.2 Behavioural ........................................................................................................... 91 3.3 Neuroimaging ....................................................................................................... 93 4. Discussion ................................................................................................................ 100 10 Chapter 5. Reward Valuation and Incentive Motivation in Addictive Disorders 1. Introduction .............................................................................................................. 105 2. Materials and Methods ............................................................................................. 110 2.1 Participants ......................................................................................................... 110 2.2 Behavioural Task ................................................................................................ 111 2.3 Self-Report Measures ......................................................................................... 113 2.4 Analysis .............................................................................................................. 113 3. Results ...................................................................................................................... 114 3.1 Self-Report Data ................................................................................................. 114 3.2 Response Time Behavioural Analysis ................................................................ 116 3.3 Subjective Ratings .............................................................................................. 118 3.4 Correlation Analyses .......................................................................................... 118 4. Discussion ................................................................................................................ 120 Chapter 6. Overlapping Decline in Orbitofrontal Grey Matter Volume Related to Cocaine Use and Body Mass Index 1. Introduction .............................................................................................................. 124 2. Materials and Methods ............................................................................................. 125 2.1 Participants ......................................................................................................... 125 2.2 Imaging Procedures ............................................................................................ 127 3. Results ...................................................................................................................... 128 4. Discussion ................................................................................................................ 132 Chapter 7. General Discussion 1. Summary .................................................................................................................. 135 2. Links to Personality Traits ....................................................................................... 143 3. Synthesis................................................................................................................... 148 4. Future Directions ...................................................................................................... 153 5. Conclusions and Implications .................................................................................. 155 References ....................................................................................................................... 157 Appendices ...................................................................................................................... 182 11 12 Chapter 1. Introduction Chapter 1. General Introduction Addictive disorders are a major mental health concern, with roughly 300,000 individuals in the UK currently dependent on an illicit substance (National Treatment Agency for Substance Misuse, 2012). The economic costs of this disease are estimated to reach fifteen billion pounds every year in health care, treatment services, law enforcement, lost productivity, and drug-related crime (Gordon, 2006). However, with almost three million people reporting having used an illicit substance in the last year, it appears that only ten percent of individuals who try drugs will become dependent upon them. The question thus arises, what are the risk and protective factors involved in these odds? How can nine out of ten individuals try drugs of abuse without developing a dependence upon them, and what is different in those one in ten who do become addicted? 1. Definition of Addiction Drug addiction is a relapsing and remitting disorder characterized by the compulsive seeking and taking of a substance, despite severe negative consequences such as poor health, legal and financial problems, and the damaging of social relationships. This includes a loss of control over use of the drug, consuming greater quantities and using more frequently than intended or despite a desire to stop, and a strong feeling of negative affect, including distress, anxiety and dysphoria when experiencing withdrawal (American Psychiatric Association, 2000). Intense feelings of craving consisting of a powerful urge and desire to use frequently accompany physical and psychological dependence, and are often triggered by associated environmental stimuli, such as certain people or places, or drug-related paraphernalia. 13 Chapter 1. Introduction Originally begun as a hedonic choice, drug taking can devolve into a compulsive habit where the behaviour is maintained despite a loss of the rewarding sensations first associated with the action. The initial positive reinforcement for drug taking comes from the pleasure derived from the euphoric ‘high’ effects of the stimulant itself, while negative reinforcement maintains continued drug-seeking behaviours, attempting to stave off withdrawal and the associated negative affect that can follow (Koob & Le Moal, 2001; Solomon, 1980). Positive reinforcers from the drug producing pleasurable experiences are followed by disparate effects in an opponent-process model, generating contrasting negative feelings in a simple dynamic control system (Solomon, 1980). These withdrawal symptoms typically manifest as opposing physiological and psychological reactions to those experienced during the initial high, motivating users to take more of the drug to alleviate the unpleasant symptoms. 2. Striatal Dopamine System and Bottom-Up Motivation All drugs of abuse act on the mesolimbic dopamine system, impacting it either directly or indirectly (Di Chiara, 1999; Koob & Nestler, 1997; Nutt, 1996). Stimulant drugs like cocaine and amphetamine directly alter ventral striatal dopamine levels by interfering with monoamine transporters, inhibiting the reuptake of endogenous dopamine from the synapses, or by increasing dopamine concentration and release in presynaptic vesicles. Psychostimulants also significantly elevate dopamine levels in the prefrontal cortex via their action on noradrenergic neurons (Di Chiara, 1999; Tanda, Pontieri, Frau, & DiChiara, 1997). Other substances like cannabis and opiates affect the system indirectly, with cannabinoid receptors and opioid peptides synapsing onto dopaminergic neurons in the nucleus accumbens shell and ventral tegmental area, causing a cascade of dopamine release throughout the mesolimbic system (Tanda, Pontieri, & DiChiara, 1997). 14 Chapter 1. Introduction Dopamine is crucially involved in reward valuation and reinforcement learning, as well as the development of habits (Baldo & Kelley, 2007; Di Chiara, 1999; Wise & Bozarth, 1985; Wise, 2004). As such, the mesolimbic dopamine system is not limited to the rewarding sensations associated with drugs of abuse, but also plays a reinforcing role in all pleasurable experiences. This includes anticipation and receipt of food, sex and money (Deadwyler, 2010; Everitt, 1990; Hernandez & Hoebel, 1988; Schott et al., 2008; Wise & Rompre, 1989), with dopaminergic activity underlying the positive reinforcing properties of both intrinsically rewarding and conditioned stimuli (Di Chiara, 1999; Wise, 2004). Dopamine agonists such as amphetamine magnify the reinforcing effects of conditioned stimuli that already depend on dopamine activity, and this enhancement makes stimulant drugs particularly susceptible to abuse and the development of compulsive tendencies. 2.1 Incentive Salience Elevation of dopamine levels in the striatum can also create associations between drug cues and the ‘high’ experienced upon administration. This enhances the substance’s abuse potential, potentiating relevant stimulus-reward associations. The anticipation of receiving the drug can then cause similar increases in dopamine release, even before the substance’s excitatory properties are experienced (Berridge & Robinson, 1998; Di Chiara, 1999; Ito, Dalley, Howes, Robbins, & Everitt, 2000; Koob & Le Moal, 2001). A formerly neutral accompaniment can thus be rendered desirable or rewarding through its association with the drug and its acute effects, initiating a transfer of incentive salience and phasic dopamine release to the associated cue (Ito, Dalley, Robbins, & Everitt, 2002; Schultz, Dayan, & Montague, 1997). Administration of psychostimulants can increase acquisition of conditioned reinforcement learning, potentiating responding for a conditioned reinforcer, as well as enhancing pavlovian-instrumental transfer and certain forms of conditioning (Everitt & Robbins, 2005; Taylor & Robbins, 1986; Wise, 2004; 15 Chapter 1. Introduction Wyvell & Berridge, 2001). Conversely, dopamine receptor antagonists diminish incentive-motivation and may impede conditioning (Koob & Le Moal, 2001; Wise, Spindler, DeWit, & Gerberg, 1978; Wise, 2004). Thus, the reinforcing qualities of drugs of abuse are thought to be facilitated through both direct and indirect activation of the mesolimbic dopamine circuit, eliciting positive feelings of reward and incentivising future use, instilling the substance and its associated cues with particular potency (Di Chiara, 1999; Koob & Le Moal, 2001). Learned drug associations can increase the likelihood for relapse long after acute withdrawal effects have dissipated. This occurs by triggering anticipatory or craving sensations upon encounter with environmental cues that were previously associated with drug administration. These stimuli have been engendered with greater incentive salience by associative learning, granting them many of the same reinforcing properties of the drug itself. These secondary drug cues can then cause a release of striatal dopamine themselves, independent of the presence of the original substance, thus hypothetically instigating anticipation and desire for the subsequent effects of the drug that typically follow (Berridge & Robinson, 1998). Feelings of craving precipitated by exposure to drug-related cues and memories have been shown to be accompanied by greater activation in the prefrontal cortex, dorsal striatum and regions implicated in the limbic system (Childress et al., 1999; Daglish et al., 2001; Garavan et al., 2000; Grant et al., 1996; Volkow et al., 2006, 2010). 2.2 Transition from Goal-Directed to Habitual Drug Behaviour There are also changes in dopamine firing within the basal ganglia that mediate the transition from initially goal-directed or hedonically motivated drug taking to more compulsive drug-related habits. This is theorized to involve a signalling transfer shift 16 Chapter 1. Introduction from ventral to dorsal striatal control, which subserves a devolution from action-outcome to stimulus-response behaviours (Belin & Everitt, 2008; Everitt & Robbins, 2005; Everitt et al., 2008; Robbins, Gillan, Smith, de Wit, & Ersche, 2012). As drug taking is devalued through a loss of the initial subjective pleasure experienced upon administration, drug seeking can become compulsive and habitual, triggered by salient cues like environment or drug-related paraphernalia. These behaviours are thought to be perpetuated by signalling from the lateral caudate and putamen, which contribute to sustained movement and habitual actions (Everitt et al., 2008; Yin, Knowlton, & Balleine, 2004; Yin, Ostlund, & Balleine, 2008). 3. Dopamine System Abnormalities in Stimulant Dependence Stimulant-dependent individuals have known impairments in fronto-striatal functioning, which are potentially exacerbated by stimulant drugs’ manipulation of the dopamine system. Alterations in cortico-striatal circuitry include reduced baseline levels of endogenous dopamine, lower dopamine D2 receptor availability, diminished dopamine transporter activity, and eventually a decline in dopamine receptor sensitivity through a down-regulation of the system via over-stimulation from prolonged drug administration (Dalley et al., 2007; Lee et al., 2009; Martinez et al., 2004, 2009; Porrino, Daunais, Smith, & Nader, 2004; Volkow et al., 1997). These can all contribute to dysfunctional patterns of responding and impulsive behaviour typically seen in stimulant-dependent individuals. For example, diminished dopamine release in the nucleus accumbens in response to methylphenidate has been seen in cocaine-dependent individuals as compared with healthy controls, and decreased dopamine receptor binding has been reported in stimulant-dependent patients (Martinez et al., 2004, 2009; Volkow et al., 1997, 2001, 17 Chapter 1. Introduction 2007). Additionally, lower endogenous dopamine receptor density in healthy individuals make them more likely to rate the stimulating effects of amphetamine as pleasant, compared with reports of noxious or over-arousing and anxiety-inducing effects in those with naturally higher levels (Thanos et al., 2001; Volkow et al., 1999). Performanceenhancing stimulant drugs are also most effective in individuals with higher baseline rates of impulsivity, which has been linked to reduced dopamine receptor availability (Cools, Sheridan, Jacobs, & D’Esposito, 2007). Thus, subjective pleasure experienced from stimulant drugs, as well as their abuse potential, can largely depend on an individual’s underlying dopamine activity (Volkow et al., 2006). This has led to the self-medication hypothesis for the initiation and eventual compulsive continuation of stimulant use in dependent individuals, presumably subconsciously ‘treating’ their low endogenous dopamine activity with psychostimulant drugs. There have also been reports of structural abnormalities in the basal ganglia in stimulantdependent individuals, specifically increased grey matter volume in the caudate, putamen and pallidum (Chang et al., 2005; Ersche et al., 2011; Jacobsen, Giedd, Gottschalk, Kosten, & Krystal, 2001). Differences in basal ganglia volume have also been linked to attention deficit/hyperactivity disorder (ADHD) (Castellanos et al., 1996), with decreases in striatal volume thought to potentially underlie the pathological impulsive responding. The finding of similar rates of impulsivity in patients with ADHD as in those with stimulant-dependence, as well as similar treatment options using dopamine and noradrenaline agonists, provides further support for the self-medication hypothesis in stimulant dependence (Vaidya et al., 1998). 18 Chapter 1. Introduction 3.1 Genetic Variants Implicated in Addiction These findings may be modified by a genetic profile affecting dopamine pathways, placing individuals at a greater susceptibility for reward system dysfunction. Due to its role in dopamine receptor availability, the A1 allele of the TaqIA gene has historically been a target for drug and alcohol research over the last 20 years (Hall, Drgonova, Jain, & Uhl, 2013; Noble, 2000). Presence of the A1 allele can cause a 30-40% reduction in striatal dopamine D2 receptors (Jonsson et al., 1999), and has been implicated in corresponding deficits in glucose metabolism in the orbitofrontal cortex (OFC), frontal and temporal gyri, insula, hippocampus, and dorsal and ventral striatum (Noble, Gottschalk, Fallon, Ritchie, & Wu, 1997). As such, some studies have reported that chronic drug users show a greater preponderance of A1 compared to the general population, and its presence is thought to underlie some of the structural and behavioural abnormalities seen in dependent individuals (Comings, Muhleman, Ahn, Gysin, & Flanagan, 1994; Noble, 2000). Another possible genetic target for the dopamine system’s role in addiction that has received significant attention is the dopamine transporter gene (DAT) (Guindalini et al., 2006; Uhl, Hall, & Sora, 2002). However, it should be noted that research has veered away from dopamine gene expression theories in recent years, and critiques about prior genetic research in addiction have been raised (Hall et al., 2013). 4. Impulsivity Decreased striatal D2 receptor availability is frequently cited as a risk factor in stimulant dependence, as it has been empirically linked to an increased tendency for impulsive behaviours (Dalley, Everitt, & Robbins, 2011; Dalley et al., 2007; Kirby & Petry, 2004; Lee et al., 2009; Perry & Carrol, 2008; Volkow et al., 1993, 1996, 2001). Impulsivity is the tendency to act prematurely, without foresight, and despite adverse consequences 19 Chapter 1. Introduction (Robbins et al., 2012). Conversely, sensation-seeking has been defined as an interest in searching out new experiences, even if they are risky or potentially dangerous. Trait impulsivity is highly implicated in addictive tendencies, often divided into difficulties with waiting or delaying gratification, and problems with stopping a prepotent response (Dalley et al., 2011; Perry & Carrol, 2008). This places impulsive individuals at a disadvantage in inhibiting maintained actions or urges, as well as potentially increasing their susceptibility to the immediate rewarding properties of drugs of abuse over consideration of long-term consequences. Increases in impulsivity are therefore particularly associated with the acquisition of drug-taking behaviours, as well as the loss of control seen during the transition from goal-directed to compulsive drug use (Perry & Carrol, 2008; Everitt et al., 2008). In animal models, Dalley and others have shown a greater preference for to cocaine and an increase in self-administration rates in highly impulsive animals, thought to correspond with a decrease in D2 receptor binding availability in the ventral striatum (Dalley et al., 2007). Increases in sensation- or novelty-seeking in rats has also been linked to a higher proclivity for initial stimulant administration, whereas impulsivity as a distinct trait is more associated with the development of compulsive drug behaviours (Belin, Mar, Dalley, Robbins, & Everitt, 2008). High impulsivity has also been linked to greater risk for relapse after drug abstinence (Everitt et al., 2008). Similarly, high levels of impulsivity and impairments in inhibitory control, as measured by self-report assessments and behavioural tasks, are associated with increased severity in human drug dependence (Clark, Robbins, Ersche, & Sahakian, 2006; Ersche, Roiser, Robbins, & Sahakian, 2008). The most commonly administered self-report measure for impulsivity is the Barratt Impulsivity Scale (BIS-11), a 30-item questionnaire assessing 20 Chapter 1. Introduction subjective tendencies towards impulsive-related actions, such as frequent moves or job changes, and self-control abilities like paying attention, concentrating and future planning (Patton, Stanford, & Barratt, 1995). Items are divided into three subscales: attention, motor behaviour and non-planning. Behaviourally, impulsivity can be assessed with either ‘stopping’ or ‘waiting’ paradigms. This is typically done using the Stop Signal Reaction Time Task (SSRTT), a behavioural test assessing stopping ability as an indicator of impulsivity and poor motor inhibitory control (Ersche et al., 2011; Logan, Schachar, & Tannock, 1997; Robbins et al., 2012). In the task, the participant must respond rapidly to a repeated visual stimulus (i.e., pressing a button every time an arrow appears on the screen); however, they must inhibit this ongoing response when they are presented with a stop signal, such as a loud beep. Successful stops indicate adequate inhibitory control functioning, whereas impairments are suggestive of ‘stopping impulsivity’. Similarly, cognitive control is often measured using a Stroop task. Here, participants must name the font colour of a target word that spells out either the same or a different colourword as the font (MacDonald, Cohen, Stenger, & Carter, 2000; Stroop, 1935). Responses to incongruent colour-word combinations present a greater cognitive demand than congruent pairings because of the interference of the prepotent tendency to read a word rather than determine its colour. The interference score indexes how well a person exerts cognitive control over this automatic behaviour (word-reading) in favour of a more unusual behaviour (colour-naming). The task can also be adapted to measure emotional interference causing attentional bias (i.e., for phobias or drug-related material in substance users). 21 Chapter 1. Introduction Waiting or choice impulsivity can be assessed with delay discounting measures, in which the participant must choose between a small immediate reward and a larger delayed option (Kirby & Petry, 2004). Impulsive individuals often over-value the smaller sooner option, unable to wait and devaluing the larger delayed reward. Stopping and waiting are thought to be two distinct aspects of the generalised impulsivity umbrella, and both of these constructs only correlate mildly with self-report ratings (Reynolds, Ortengren, Richards, & de Wit, 2006). An illuminating study by Lee et al. (2009) successfully synthesised these concepts of underlying biological and personality risk-traits for dependence with the consequential effects of stimulant use. Elevated BIS-11 self-report impulsivity scores in methamphetamine users were related to decreased dopamine D2/D3 receptor binding in the caudate and putamen, and both measures were significantly influenced by lifetime exposure to methamphetamine (Lee et al., 2009). Additionally, poor behavioural inhibitory performance on the SSRTT has been identified as a predictive factor for drug and alcohol related problems in adolescents (Nigg et al., 2006; Whelan et al., 2012). Together, this evidence suggests that there are potentially both predating differences in dopamine system functioning, with related elevations in impulsivity in those who become dependent on stimulants, as well as potential additional exacerbatory effects on the dopamine system and impulsive behaviour from prolonged use of the drug itself (Ersche, Bullmore, et al., 2010). 5. Prefrontal Cortex and Top-Down Control In addition to dopamine-related abnormalities in the striatum, structural and functional aberrations in the prefrontal cortex (PFC) have been implicated in stimulant dependence. Decreases in D2 receptor availability in the PFC have been reported in stimulant22 Chapter 1. Introduction dependent individuals, with correlations seen between striatal and prefrontal dopamine functioning (Schoenbaum & Shaham, 2008; Volkow et al., 1993, 2001). This suggests an important feedback loop between the striatum and PFC, modulated by dopamine and potentially malfunctioning in stimulant-dependent individuals. Porrino and colleagues have shown metabolic changes in both cortical and subcortical limbic regions accompanying acute cocaine exposure in non-human primates (Porrino, Smith, Nader, & Beveridge, 2007). This includes decreases in ventral striatal and ventromedial prefrontal cortex glucose metabolism (Porrino et al., 2002). These effects were exacerbated by prolonged stimulant administration, with both more severe and more widespread disruption seen throughout these regions in association with chronic use (Beveridge, Smith, Daunais, Nader, & Porrino, 2006). Advancing changes into the dorsal striatum with long-term exposure was also evident, providing support for the proposed shift in dopamine signalling control from the ventral to the dorsal striatum, as previously suggested (Everitt & Robbins, 2005). Abnormalities in prefrontal structural integrity have also been associated with prolonged stimulant use, including significant reductions in both grey matter volume and white matter connectivity. This finding has been reliably reproduced in numerous samples of both abstinent and currently dependent stimulant users from a variety of different groups (Ersche et al., 2011; Franklin et al., 2002; Lim, Choi, Pomara, Wolkin, & Rotrosen, 2002; Liu, Matochik, Cadet, & London, 1998; Matochik, London, Eldreth, Cadet, & Bolla, 2003; Tanabe et al., 2009). The cognitive ramifications of these structural differences are listed below. 23 Chapter 1. Introduction 5.1 Cognitive Impairment The prefrontal cortex is critically involved in self-control and impulsivity, as well as goal representation, making dysfunction in this area especially significant. Thus, these structural changes can have considerable consequences, and the PFC abnormalities cited above are thought to be at the root of many of the cognitive difficulties seen in drugdependent individuals. Several decades of work have reported on a wide-range of cognitive deficits observed in stimulant-dependent individuals (Ersche & Robbins, 2011; Goldstein & Volkow, 2011; Rogers & Robbins, 2001; Verdejo-Garcia, Bechara, Recknor, & Perez-Garcia, 2006; Verdejo-García & Pérez-García, 2007). This includes crucial difficulties with response inhibition and self-control (Goldstein, Volkow, Wang, Fowler, & Rajaram, 2001; Hester & Garavan, 2004; Kaufman, Ross, Stein, & Garavan, 2003; Monterosso, Aron, Cordova, Xu, & London, 2005; Verdejo-Garcia, Perales, & Perez-Garcia, 2007), as well as detriments in working memory (Ersche, Clark, London, Robbins, & Sahakian, 2006; Tomasi et al., 2007), decision-making (Bechara & Damasio, 2002; Bechara, 2005; Bechara et al., 2001; Ersche et al., 2005; Ersche, Fletcher, et al., 2006), sustained attention (Gooding, Burroughs, & Boutros, 2008; Horner, 1999; Levine et al., 2006; London et al., 2005), task-switching (Ersche et al., 2008), and affective responding and emotion regulation (Fox, Axelrod, Paliwal, Sleeper, & Sinha, 2007). These impairments often correlate with years of substance use, implicating prolonged exposure to stimulant drugs in more severe dysfunction. However, other studies have shown executive function difficulties in the drug-naïve relatives of dependent users, including both biological siblings and offspring, suggesting that these impairments may also be underlying risk factors for dependence (Ersche, Turton, et al., 2012; Silveri, Rogowska, McCaffrey, & Yurgelun-Todd, 2011). 24 Chapter 1. Introduction 6. Orbitofrontal Cortex A ventromedial component of the PFC, encompassing medial areas of the orbitofrontal cortex (OFC) and stretching back into the anterior cingulate cortex (ACC), has been particularly implicated in stimulant dependence. The OFC is defined as the anterior portion of Brodmann areas 10 and 11, wrapping medial to lateral; areas 12 and 25 more medially and caudally; and laterally extending posteriorly into area 47 (Elliott, Dolan, & Frith, 2000). The medial OFC – often synonymous with the ventromedial prefrontal cortex, an area overlapping both structurally and functionally (Noonan, Mars, & Rushworth, 2011; Rushworth, Noonan, Boorman, Walton, & Behrens, 2011) – is critical in reward valuation, affective decisionmaking and goal-directed behaviour (Gottfried, O’Doherty, & Dolan, 2003; Rolls, 2000; Schoenbaum, Roesch, & Stalnaker, 2006; Schoenbaum & Shaham, 2008; Volkow & Fowler, 2000). This is thought to be due in part to the region’s projections to and from limbic regions, including the ventral striatum and basolateral amygdala (Elliott, Dolan, & Frith, 2000; Rushworth, Behrens, Rudebeck, & Walton, 2007; Schoenbaum et al., 2006). This is distinct from the executive control functions and ‘colder’ cognitive processes of the more lateral, posterior and superior regions of the prefrontal cortex. Indeed, the more lateral regions of the OFC are typically associated with suppressing previously initiated responses, as in the case of reversal learning (Elliott et al., 2000). The lateral OFC has also been linked to the processing of both positive and negative feedback, whereas the medial OFC is most strongly implicated in reward learning (Noonan et al., 2011). There is also evidence that the lateral OFC is involved in compulsive behaviours, such as in obsessive-compulsive disorder (Joel, Doljansky, Roz, & Rehavi, 2005). 25 Chapter 1. Introduction In the current thesis, I am most concerned with the ventromedial prefrontal cortex/medial orbitofrontal cortex and this region’s role in reward processing and affective decisionmaking; and in discussion of the OFC, this is the region being referred to unless otherwise specified. 6.1 OFC Abnormalities in Stimulant Dependence Similar structural abnormalities as those seen in the wider prefrontal cortex in dependent stimulant users have been reported in the OFC, including a reduction in glucose metabolism relating to dopamine receptor availability in the striatum, as well as significant decreases in medial OFC grey matter volume in chronic drug users (Nader, Czoty, Gould, & Riddick, 2008; Porrino et al., 2007; Volkow et al., 1997, 2001). Using voxel-based morphometry analysis, these decreases in OFC grey matter volume and connectivity have been linked to increases in drug-related compulsivity, as determined by the Obsessive-Compulsive Drug Use Scale (OCDUS) (Franken, Hendriksa, & van den Brink, 2002), with greater disruption in the OFC associated with higher compulsivity ratings (Ersche et al., 2011; Meunier et al., 2011). Conversely, enlarged basal ganglia volume, particularly in the left caudate nucleus, was related to elevated impulsivity levels in these individuals (Ersche et al., 2011). 6.2 Reward Valuation Activation of the OFC is associated with the expected value of an outcome, and neurons in the OFC exhibit greater firing for a preferred reward, with the amplitude of the cell’s response dependent on the subjective value of the anticipated receipt (Gottfried et al., 2003; Schoenbaum et al., 2006; Tremblay & Schultz, 1999). This subjective reward valuation includes a comparison of outcomes of different natures and over different timecourses, and is a changing and transient response dependent on an individual’s current 26 Chapter 1. Introduction state and motivations (Levy & Glimcher, 2012; Rolls, 2000). These anticipatory reward valuations are learned over time through past experiences and, combined with feedback processing via the anterior cingulate cortex, are crucial in the process of affective decision-making (Elliott et al., 2000; Rushworth et al., 2007; Rushworth & Behrens, 2008). Consequently, abnormal or reduced medial OFC functioning has been linked to impaired decision-making in a variety of paradigms and patient groups (Bechara, Damasio, Damasio, & Anderson, 1994; Bechara, Damasio, & Damasio, 2000; Clark et al., 2008; Grant, Contoreggi, & London, 2000). In dependent drug users this effect is particularly evident, and it is thought to partially underlie the known risky behaviours that often accompany chronic drug taking (Bechara & Damasio, 2002; Ersche & Robbins, 2011). This includes needle sharing, unsafe sexual behaviour and driving while intoxicated (Aitken, Kerger, & Crofts, 2000; Albery, Strang, Gossop, & Griffiths, 2000; Cavazos-Rehg et al., 2009; Hwang et al., 2000), actions that are indicative of potential decision-making impairments. In addition to the structural and metabolic reductions seen in the OFC in drug-dependent individuals, decreased functional activation in response to cognitive tasks has also been reported in a number of studies (Bolla et al., 2003; Ersche et al., 2011; London, Ernst, Grant, Bonson, & Weinstein, 2000; Matochik et al., 2003; Schoenbaum & Shaham, 2008; Volkow et al., 1993). This includes dampened responding to universal reinforcers like food, money and sex (Garavan et al., 2000; Goldstein & Volkow, 2011). In one study, cocaine-dependent individuals showed reduced functional magnetic resonance imaging (fMRI) blood oxygen level dependent (BOLD) responding in the OFC in response to a monetary reward compared with control participants, and they also demonstrated a lack of sensitivity to different reward magnitudes (Goldstein, Tomasi, Alia-Klein, et al., 2007; Goldstein et al., 2007). However, increased activation in this same region is also present 27 Chapter 1. Introduction in response to drug-related stimuli in dependent users, which is often implicated in maintaining or rekindling drug-seeking behaviour, even despite a desire to stop (Childress et al., 1999; Schoenbaum & Shaham, 2008; Tiffany & Carter, 1998; Volkow & Fowler, 2000). This heightened response is suggestive of drug craving and a ‘hijacking’ of the reward system by prolonged stimulant use in dependent individuals (Ehrman, Robbins, Childress, & O’Brien, 1992; Garavan et al., 2000; Volkow & Fowler, 2000). Supporting this theory, in a forced choice task cocaine-addicted animals consistently chose drug rewards over a food option (Aigner & Balster, 1978; Woolverton & Anderson, 2006), and a separate study has shown elevated OFC and striatal responses following the administration of cocaine compared with receipt of a juice reward in non-human primates (Opris, Hampson, & Deadwyler, 2009). 6.3 Attentional Bias In human drug users, heightened drug-related salience is often assessed through attentional bias tasks, measuring emotional interference from associated cues. As mentioned above, an emotional Stroop task can be used to assess drug-related distraction and cognitive interference. In previous studies, greater impairment on the cocaine-word Stroop has been related to abnormal responding in the medial OFC and ACC, areas implicated in craving, reward and attention (Ersche, Bullmore, et al., 2010; Franken, 2003; Goldstein, Tomasi, Rajaram, et al., 2007). Franken (2003) posits that this biased attention network in drug users stems from dysfunctional involuntary reactions to drugrelated cues. Due to the limited nature of the brain’s attentional capacity, attention is typically allocated to only a subset of external stimuli to avoid overstimulation. However, when a stimulus is particularly potent it can ‘hijack’ this system and assume a greater proportion of the attentional resources. Modelled in an emotional Stroop task, this results in higher response latencies for salient words, as too much attention is paid to the content 28 Chapter 1. Introduction of the word rather than the colour of the font, distracting the individual from the task athand (Field & Cox, 2008; Hester, Dixon, & Garavan, 2006). Elevated activation in limbic regions, including the striatum, amygdala, ACC and OFC, is also associated with increased craving in response to drug-related cues (Childress et al., 1999; Daglish et al., 2001; Daglish & Nutt, 2003; Garavan et al., 2000; Grant et al., 1996; Volkow et al., 2006). However, purposeful cognitive inhibition of craving upon cue exposure can result in a decrease in glucose metabolic activity in these regions (Volkow et al., 2010). Therefore, although these responses are automatic and unintentional – similar to the prepotent urge to read the content of a word rather than name the colour of the font – they appear to be able to be controlled at times through cognitive effort and resistance. 6.4 Compulsive Behaviour The lateral OFC is also central in obsessive-compulsive disorder and is theorized to be linked to the compulsive nature of substance dependence. This is due to its possible involvement in habit development and updating goal-directed information; impairments in these processes are thought to be present in individuals with greater compulsive tendencies (Balleine & O’Doherty, 2010). This is most often demonstrated through reversal-learning or set-shifting tasks, where a previously rewarded response is devalued while the alternate option becomes the target. Individuals with OFC damage or dysfunction are frequently impaired on reversal-learning assessments, perseverating on the previously reinforced behaviour and failing to update their responses (Cools, Clark, Owen, & Robbins, 2002; Izquierdo, Suda, & Murray, 2004). Devaluation or aversive learning appears to be similarly impaired in animals with OFC lesions, with a failure to devaluate a stimulus-response association even after a punishment or noxious pairing (Izquierdo et al., 2004; Schoenbaum et al., 2006). These effects have been related to 29 Chapter 1. Introduction prolonged drug exposure in animal models (Jentsch, Olausson, De La Garza, & Taylor, 2002; Schoenbaum & Shaham, 2008; Schoenbaum & Setlow, 2005), and are also evident in human substance users (Ersche et al., 2008), with dependent individuals continuing to use even in the face of severe negative consequences. 7. Reward System Abnormalities As discussed above, in addition to the cognitive impairments seen in drug-dependent individuals, there is also dysfunction present in reward valuation caused by abnormalities in striatal dopamine system functioning, as well as in the ventromedial prefrontal cortex / orbitofrontal cortex. The brain’s reward system synthesizes both the bottom-up and topdown inputs from the ventral striatum, orbitofrontal cortex and anterior cingulate cortex to create an integrated view of the subjective value of various stimuli. This evaluation is often based on previous experiences, forming both explicit and implicit recollections and shaping future decisions, resulting in reinforcement-guided choices (Rushworth & Behrens, 2008). However, when this evaluative process is disrupted, as through prolonged exposure to stimulant drugs, decision-making can become impaired (Bechara, 2005; Schoenbaum et al., 2006). There are currently two well-accepted, yet conflicting theories of potential abnormal reward processing in addiction involving the responses to organically salient rewards. One, the reward deficiency theory (Bjork, Smith, & Hommer, 2008; Blum et al., 2000), premises that individuals at risk for substance abuse maintain a generally underactive reward system due to a pre-morbid reduction in the number of D2 dopamine receptors available in the mesolimbic circuit, particularly in the striatum. At-risk individuals then ‘self-medicate’, seeking out drugs and other stimulating activities to offset this imbalance and artificially elevate their naturally low levels of striatal dopamine. Additional down30 Chapter 1. Introduction regulation of these dopamine receptors due to increased dopamine turnover in the mesolimbic reward pathway after prolonged drug exposure further compacts this problem by de-sensitizing the individual and lowering the homeostatic threshold, thereby requiring greater reward salience to achieve activation (Bjork et al., 2008; Koob et al., 2004; Volkow, Wang, Fowler, & Telang, 2008; Wang et al., 2001; Wang, Volkow, Thanos, & Fowler, 2004). This further diminishes the already reduced effect of naturally rewarding stimuli to signal the release of dopamine, causing the system involving both the OFC and striatum to be under-responsive to non-drug rewards (Bjork et al., 2008; Koob & Le Moal, 2005). This decrease in D2 receptor availability is also associated with diminished OFC metabolism (Volkow et al., 2001; Volkow, Wang, Telang, et al., 2008), which is linked to both objective and subjective reward valuation and decision-making, and where impairment can result in impulsive actions (Bechara, 2005; Goldstein, Tomasi, AliaKlein, et al., 2007; Goldstein et al., 2007; Knutson, Westdorp, Kaiser, & Hommer, 2000; Robbins, Ersche, & Everitt, 2008). Conversely, the opponent-process, or incentive sensitization, theory proposes that drug users are overly sensitive to rewards, manifested in impulsive and sensation-seeking behaviour (Robinson & Berridge, 1993). Immediate rewards are disproportionately valued via delay discounting, and the ventral striatum is overly activated in response to salient stimuli. These individuals may also experience an up-regulation in striatal dopamine receptor sensitivity after excess activation of the reward pathways as a consequence of drug use, rendering them increasingly more responsive to both anticipation and receipt of reward, regardless of type. Additionally, these individuals typically have deficient or under-utilized prefrontal cortices, making self-control and impulse restriction even more challenging (Bechara, 2005; Bjork et al., 2008; Goldstein & Volkow, 2002). 31 Chapter 1. Introduction 8. Endophenotype Research For all of these abnormalities, there is an ongoing debate as to whether the cortical and subcortical aberrations present in stimulant dependence are predating risk factors for addiction or are the consequence of protracted stimulant use, resulting in potential neurotoxic effects on the brain. This includes both the alterations in reward sensitivity subserved by the dopamine system and the suboptimal frontal cortex control circuitry. Research from animal models provides strong evidence for both the chicken and the egg argument, with underlying predefined personality traits, such as impulsivity and sensation-seeking, implicated in a greater risk for both the initiation and development of compulsive or addictive drug behaviours (Belin et al., 2008; Dalley et al., 2007). However, there is also strong evidence for further disruption in the fronto-striatal dopamine circuitry, particularly involving structural abnormalities in the prefrontal cortex, that are associated with prolonged drug abuse and the devolution into compulsive drug-seeking and taking (Porrino et al., 2007). In human participants, one of the few means to investigate this chicken-or-egg debate is through endophenotype research. Endophenotypes are stable quantifiable variables associated with the genetic risk for a disorder and that are abnormal both in patients and their relatives (Gottesman & Gould, 2003; Robbins et al., 2012). This includes both the structural and behavioural abnormalities typically associated with the phenotype for a pathology, such as the increased impulsivity and reduced frontal cortical volume implicated in stimulant dependence. If there is evidence of impairment in both the patients and their non-drug using biological relatives, it suggests an underlying risk factor for dependence. However, greater dysfunction present only in the patient group is indicative of potentially exacerbated abnormalities from the chronic stimulant use itself, or perhaps an additional predisposing element unique to this group. 32 Chapter 1. Introduction A new line of research from our group has attempted to address this question by studying stimulant-dependent individuals, their non-dependent biological siblings and unrelated healthy control volunteers on a variety of cognitive, personality and structural imaging assessments (Ersche, Jones, et al., 2012; Ersche, Turton, et al., 2012; Ersche, Turton, Pradhan, Bullmore, & Robbins, 2010). In an initial report, higher levels of trait impulsivity, as measured by the BIS-11, and sensation-seeking, assessed using the Zuckerman Sensation Seeking Scale (SSS-V) (Zuckerman, Eysenck, & Eysenck, 1978), were evident in the stimulant-dependent individuals compared with controls (Ersche, Turton, et al., 2010). However, their non-dependent siblings also showed elevated impulsivity as compared to controls, but no difference in sensation-seeking. This suggests that impulsivity may be an underlying trait predating stimulant dependence, which would indicate potential genetic or childhood environmental effects on dopamine levels in both members of the sibling pair, such as presence of the TaqIA allele. However, sensationseeking, which has been linked to the initiation of drug use and experimentation, was unique to the stimulant-dependent individuals, likely spurring their initial drug-seeking behaviour (Belin et al., 2008; Ersche, Turton, et al., 2010). An absence of this trait in the sibling participants may therefore have protected them from initiating drug use, and subsequently from following the same path as their dependent brothers and sisters. In addition to self-report trait impulsivity, performance on the SSRTT was equally poor in both stimulant-dependent individuals and their siblings as compared with healthy controls. These behavioural impairments corresponded to abnormalities in cortical and subcortical brain structure, including decreases in grey matter volume in the posterior postcentral gyrus, superior temporal gyrus and posterior insula, as well as increases in the medial temporal lobe and basal ganglia (Ersche, Jones, et al., 2012). Notably, performance on the SSRTT was also directly related to reductions in white matter 33 Chapter 1. Introduction integrity, particularly in tracts adjacent to the inferior frontal gyrus, an area important for self-control. Specifically, white matter fractional anisotropy (FA) values significantly negatively correlated with SSRTT ability, such that a decrease in FA integrity corresponded to increased time needed to stop a prepotent response. These structural impairments were evident in the stimulant-dependent and sibling participants and were significantly higher in both groups compared with control participants. These findings persuasively demonstrate shared structural and behavioural abnormalities in both stimulant-dependent individuals and their non-dependent biological siblings, providing evidence for underlying dysfunction in the self-control circuitry that predates and potentially predisposes an individual for substance abuse. However, there was also evidence of crucial differences that might have served as additional risk factors in the dependent individuals, or, conversely, protective factors in the siblings. 9. Recreational Stimulant Use It is important to note that the vast majority of individuals who use drugs do not become addicted, and of the estimated 16.2 million people who use cocaine worldwide, only one out of every eight is thought to develop dependence (United Nations Office on Drugs and Crime, 2012). Furthermore, there appears to be a unique subset of individuals who are able to maintain stable, controlled, occasional use of stimulant drugs without developing dependency or abuse. These ‘recreational users’ are thought to possess greater selfcontrol over their drug-taking behaviours. They are also potentially lacking some of the crucial risk factors involved in the development of dependence, including increases in impulsivity and compulsivity (Ersche, Jones, Williams, Smith, et al., 2013). Recent research has suggested that there are inherent differences in the brains of individuals who are able to use drugs recreationally, most notably increased volume in the orbitofrontal 34 Chapter 1. Introduction cortex compared with dependent stimulant users, as well as healthy control volunteers (Ersche, Jones, Williams, Smith, et al., 2013). Differences in key personality traits also emerged between these groups, with recreational users reporting significantly lower levels of impulsivity and compulsivity than both dependent individuals and their nondependent siblings, providing further support that these characteristics are underlying elements. However, the recreational users did crucially share elevated levels of sensationseeking with the dependent individuals, suggesting that this trait is implicated in the tendency towards initial drug experimentation, yet not in the development of dependence (Ersche, Jones, Williams, Smith, et al., 2013). Previous research into non-dependent stimulant users has been limited and the results are somewhat conflicting. This is thought to largely be due to varying definitions of the quality and quantity of use classifying an individual as ‘recreational’. Some prior studies have shown evidence of impairment on tasks of executive function in recreational users compared with controls (Colzato, Huizinga, & Hommel, 2009; Colzato, van den Wildenberg, & Hommel, 2007; Soar, Mason, Potton, & Dawkins, 2012), while other assessments showed no difference in performance (Colzato et al., 2009). If the structural and functional abnormalities reported in stimulant-dependent individuals were largely due to the effects of cocaine exposure on the brain, recreational users would be expected to show similar, though not as severe, changes in structure and function. However, they would not possess the attributes associated with increased pre-morbid risk for dependence. Furthermore, there may be additional changes in individuals who can use stimulant drugs recreationally that serve as protective factors against the development of dependence. Other key dissociations between dependent cocaine users, their nondependent biological siblings and recreational users of the drug, similar to that of impulsivity and sensation-seeking, could help elucidate the distinction between 35 Chapter 1. Introduction underlying neurobiological abnormalities associated with risk for dependence, and those traits that are consequential from the effects of cocaine on the brain. 10. Food Addiction It has been suggested that the behavioural patterns and neurochemical changes implicated in heightened risk for addiction do not just apply to drugs of abuse. Similar disadvantageous habits have been associated with problem gambling behaviour and, more recently, compulsive overeating leading to obesity (Avena, Rada, & Hoebel, 2008; Davis & Carter, 2009; Ifland et al., 2009; Parylak, Koob, & Zorrilla, 2011; Pelchat, 2002). Binge eating is defined as: “recurring episodes of eating, in a discrete period of time, an amount of food that is definitely larger than most people would eat during a similar period of time [with a]…lack of control during the episodes” (American Psychiatric Association, 2000), criteria that closely match those used to describe drug dependence (Table 1.1). Specifically, addicted individuals experience a lack of control in the face of food or drugs of abuse, have a continuation of over-use despite severe health, social, legal and financial problems, and are unsuccessful at attempts to cut back or reduce their consumption. These behaviours are typically accompanied by feelings of guilt, remorse and distress. 36 Chapter 1. Introduction Table 1.1. A categorical comparison of the DSM-IV definitions of substance dependence and criteria for binge eating behaviour Co-Morbid Symptom Substance Dependence Binge Eating Disorder Escalation of use The substance is taken in larger amounts or over a longer period than intended Eating large amounts of food when not feeling physically hungry Loss of control There is a persistent desire or unsuccessful effort to cut down or control substance use A sense of lack of control during the episodes, e.g., a feeling that one can’t stop eating or control what or how much one is eating Social consequences Important social, occupational or recreational activities are given up or reduced because of use Eating alone because of being embarrassed by how much one is eating Personal distress The substance use is continued despite knowledge of having a persistent physical or psychological problem that is likely to have been caused or exacerbated by the substance Feeling disgusted with oneself, depressed, or feeling very guilty after overeating; Marked distress regarding binge eating; Eating until feeling uncomfortably full In addition to analogous behavioural traits, there are also similarities in the brain structure and neurochemical profile of substance-dependent and obese individuals. These include abnormalities in the dopamine and opioid neurotransmitter systems, changes in frontostriatal circuitry, and associated dysfunctional impulsive and compulsive behaviours (Avena, Rada, Bocarsly, & Hoebel, 2005; Avena, Rada, & Hoebel, 2009; Volkow, Wang, Fowler, et al., 2008; Wang et al., 2004). Obese individuals show a decrease in striatal dopamine D2 receptor availability, similar to those with problem drug use (Wang et al., 2001, 2004). Highly palatable foods also directly affect the mesolimbic dopamine and opioid pathways, and cue-induced anticipation for foods high in fat or sugar can elevate 37 Chapter 1. Introduction activity in the fronto-limbic circuitry, thought to correspond with striatal dopamine release (Rada, Avena, & Hoebel, 2005; Small, Jones-Gotman, & Dagher, 2003). The reward deficiency hypothesis has thus similarly been advanced for obese individuals to explain how a baseline hypo-functioning dopamine system might lead to self-medication through over-consumption of high-fat/high-sugar foods (Davis & Fox, 2008; Davis, Strachan, & Berkson, 2004). Although the premise is still controversial (Ziauddeen & Fletcher, 2013; Ziauddeen, Farooqi, & Fletcher, 2012), it has been proposed that in instances of uncontrollable binge eating behaviour leading to obesity there are several behavioural and neurobiological parallels with drug addiction, and that using an addiction model (and similar pharmacological treatments) may help when addressing this behaviour (Cambridge et al., 2013; Giuliano, Robbins, Nathan, Bullmore, & Everitt, 2012). 11. Aims and Objectives The neurobiological underpinnings of key traits involved in drug addiction, namely impulsivity, cognitive control and reward valuation, will be compared in dependent stimulant users, their non-dependent biological siblings, recreational cocaine users, and non-drug using healthy control volunteers in an attempt to parse out the underlying risk and protective factors involved in susceptibility for stimulant dependence. If additional similarities were seen between dependent individuals and their biological siblings, it would provide further support for the notion of endophenotypes for drug dependence, with traits and behaviours implicated in addiction predating drug initiation and creating a greater risk for dependence. Conversely, greater similarities between the two drug-using groups – dependent and recreational – would suggest shared abnormalities as a result of stimulant drug use. Alternatively, unique differences in the siblings and recreational 38 Chapter 1. Introduction participants from the dependent users, and even from healthy control individuals, could indicate potential protective factors in these groups that prevented them from developing drug dependence. In Chapter 2, this will be investigated using the classic colour-word Stroop task to assess cognitive control ability and its neurobiological correlates in dependent stimulant users, their non-dependent biological siblings and healthy control volunteers. This will help determine whether impairments in cognitive control serve as an endophenotype for stimulant dependence, as difficulties with impulsivity and motor control have been previously shown to do. In Chapter 3, these cognitive control abilities will be assessed in the face of salient cues, using an emotional drug-word Stroop task during fMRI to measure attentional bias and distraction to drug cues. This will be administered to the same group of dependent stimulant users and healthy control volunteers, as well as to a group of recreational users of cocaine to determine if recreational users experience similar symptoms of craving and attentional bias as dependent individuals. In Chapter 4, responses to both drug rewards and a universal reinforcer, money, will be compared between all four groups to assess differences in behavioural and functional reward responding, and to determine its role as a potential risk or protective factor in stimulant dependence. I will also explore these traits in another potential addiction spectrum disorder, binge eating leading to obesity, comparing obese and stimulant-dependent individuals to assess the wider dimensionality and application of these neurobiological behaviours. Similar tendencies between the two groups would provide support for the notion of ‘addiction spectrum disorders’, classifying over-eating as a type of food addiction – a currently 39 Chapter 1. Introduction contentious proposal. Additionally, comparisons between these groups – both potentially types of behavioural and chemical dependencies – could provide further insight into whether the differences seen in addicted individuals are a result of the use of stimulants or are a maladaptive pattern of behaviour that go beyond drugs of abuse. In Chapter 5 the work on reward responding from Chapter 4 will be expanded to explore responding to a variety of different reward conditions in a separate group of dependent stimulant users and healthy controls, as well as compared to obese adults with binge eating disorder. In Chapter 6 grey matter volume in the orbitofrontal cortex, an area crucial for the previously explored behaviours of reward valuation, will be compared between cocainedependent individuals and healthy control participants, investigating changes in cortical volume relating to both years of stimulant use and increases in body mass index. Finally, in Chapter 7, the findings reported in the preceding chapters will be summarized and contextualized in a general discussion, remarking on the significance of these findings and the impact and possibilities for future research. 40 Chapter 2. Cognitive control endophenotype in drug dependence Chapter 2. Cognitive Control Dysfunction and Abnormal Frontal Cortex Activation in Stimulant Drug Users and their Biological Siblings 1. Introduction As outlined in the General Introduction, disability in drug-dependent individuals is commonly marked by impairments in cognitive function and concomitant neural biomarkers. This includes abnormalities in the orbitofrontal cortex (OFC) and loss of prefrontal cortex grey matter volume (Ersche et al., 2011; Franklin et al., 2002; Matochik et al., 2003; Schoenbaum & Shaham, 2008; Tanabe et al., 2009; Volkow et al., 2001), along with increases in impulsivity and poor inhibitory control (Garavan et al., 2000; Jentsch & Taylor, 1999). These deficits are typically viewed as the consequence of protracted stimulant use (Koob & Volkow, 2010), however, evidence suggests they may also predate heavy drug taking and facilitate the transition from recreational to compulsive use (Belin et al., 2008; Dalley et al., 2011; Ersche, Turton, et al., 2010; Ersche, Jones, et al., 2012). As such, these behavioural and neurological traits may serve as endophenotypes for dependence, potentially predisposing an individual for addiction. An endophenotypic trait is a stable, quantifiable biological or behavioural characteristic that is intrinsically associated with the pathology of a disorder. Importantly, it is present not only in the patient group, but also in their unaffected relatives. This suggests that the trait is heritable and possibly tied to a genetic risk for the condition, however, it is also an intermediate variable – involved in the disorder but not necessarily deterministic as it also appears in the unaffected relatives. The colour-word Stroop is a well-known test of cognitive inhibition (MacDonald et al., 2000; Stroop, 1935), assessing executive control over an automatic behaviour (word41 Chapter 2. Cognitive control endophenotype in drug dependence reading) in favour of a more unusual behaviour (colour-naming). Interference represents a conflict between the two sources of information (font colour and word meaning), and cognitive control is needed to overcome this conflict. Performance is measured by response latencies and interference scores, derived from the difference between congruent and incongruent trial response times (RTs). Greater discrepancy between these conditions indicates increased impairment. Previous research investigating Stroop performance in stimulant-dependent individuals (SDIs) using functional magnetic resonance imaging (fMRI) has shown impaired inhibition on the task, with corresponding abnormalities in brain activation (Nestor, Ghahremani, Monterosso, & London, 2011; Salo et al., 2002; Verdejo-Garcia et al., 2007). Performance has been used to successfully predict drug treatment outcomes (Brewer, Worhunsky, Carroll, Rounsaville, & Potenza, 2008; Carpenter, Schreiber, Church, & McDowell, 2006; Streeter et al., 2008), with increased activity in the prefrontal cortex (PFC), anterior cingulate cortex (ACC) and dorsal striatum positively correlated with self-reported abstinence and time spent in rehabilitation facilities (Brewer et al., 2008; Nestor et al., 2011). Other studies of the Stroop have reported no differences in behavioural performance between SDIs and controls, but have shown significantly less activation in the OFC, inferior frontal gyrus (IFG), ACC, parietal lobe, thalamus and caudate nucleus (Barros-Loscertales et al., 2011; Bolla et al., 2004; Brewer et al., 2008; Ersche, Bullmore, et al., 2010; Goldstein et al., 2001; Salo, Fassbender, Buonocore, & Ursu, 2012). However, as with most cognitive impairments in stimulant dependence, it is unknown whether these behavioural differences and corresponding neural abnormalities are due to pre- or post-morbid factors – i.e. whether they precede or are the result of long-term drug 42 Chapter 2. Cognitive control endophenotype in drug dependence taking. Comparing SDIs and their unaffected biological siblings on the colour-word Stroop could provide insight into whether the present impairments in inhibitory control are precursory or consequential to drug abuse via exploration of endophenotypes for stimulant dependence. Shared abnormalities in behavioural performance and brain activation during the task would suggest an endophenotype of impaired cognitive control and increased risk for stimulant dependence, whereas greater disability in the SDIs could indicate direct consequences of chronic stimulant use. On an assessment of Stroop performance using fMRI in SDIs, their biological siblings and unrelated healthy control volunteers, we predicted: 1) that both SDIs and their siblings would be impaired compared with controls, suggesting an endophenotype of poor cognitive control; 2) that SDIs would be more impaired than their siblings, indicating further drug-induced disability; and 3) that interference scores would correlate with abnormalities in IFG activation on the task, a region previously implicated in inhibitory control (Bunge, Ochsner, Desmond, Glover, & Gabrieli, 2001; Nestor et al., 2011; Zysset, Muller, Lohmann, & von Cramon, 2001). 2. Materials and Methods 2.1 Participants Three equal groups of 50 stimulant-dependent individuals, 50 of their non-dependent biological siblings and 50 unrelated healthy control volunteers were tested according to protocol approved by the Cambridge Research Ethics Committee (Ersche, Jones, et al., 2012; Ersche, Turton, et al., 2012, 2010). SDIs were recruited through drug-treatment centres and word-of-mouth; sibling participants were recruited through the initial contact with their dependent brother or sister. Healthy control volunteers were recruited through the community using local advertisements. 43 Chapter 2. Cognitive control endophenotype in drug dependence All participants were between the ages of 18-55, had no history of psychotic or neurodevelopmental disorder, neurological illness or traumatic head injury, and were fluent in English. Participants were screened for mental illness using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID) (First, Spitzer, Gibbon, & Williams, 2002), semi-structured interview for drug history, and checked for current physical health, colour-blindness and demographic information. Written informed consent was obtained prior to enrolment. SDIs were included in the study if they satisfied DSM-IV-TR criteria for cocaine (94%) or amphetamine (6%) dependence, had a first-degree sibling who had no personal history of drug abuse (with the exception of nicotine), shared both biological parents, and was able to take part. Control volunteers had no personal or family history of drug or alcohol dependence and were matched for age, gender and education levels. Severity of drug use in SDIs was measured using the Obsessive Compulsive Drug Use Scale (OCDUS) (Franken, Hendriksa, & van den Brink, 2002), age of onset and years of use. Drug abuse tendencies in control and sibling participants were assessed using the Drug Abuse Screening Test (DAST-20) (Skinner, 1982). Alcohol use and depression levels were measured in all participants using the Alcohol-Use Disorder Identification Test (AUDIT) (Saunders, Aasland, Babor, Delafuente, & Grant, 1993) and Beck Depression Inventory (BDI-II) (Beck, Steer, & Garbin, 1988); IQ levels were estimated with the National Adult Reading Test (NART) (Nelson, 1982). See Table 2.1 for demographic information. Twelve individuals were excluded due to head movement (greater than 1.5 mm in any direction, or enough to cause inter-slice variance), the presence of a clinically significant structural abnormality, or inadequate task performance in which blocks of trials had to be discarded. This resulted in 138 total participants: control=47, SDI=42, sibling=49. 44 Chapter 2. Cognitive control endophenotype in drug dependence Table 2.1. Demographic information and ANOVA group comparisons for 42 stimulant-dependent individuals, 49 of their biological siblings and 47 unrelated healthy control volunteers Control Mean (SD) Sibling Mean (SD) SDI Mean (SD) Demographics Age 32.34 (8.63) 32.63 (8.35) 34.24 (7.39) Sex (% male) 63.8% 49.0% 95.2% IQ (NART) 112.67 (8.09) 108.91 (8.88) 110.64 (7.46) Education (years) 12.70 (1.92) 12.12 (2.00) 11.69 (1.70) Depression (BDI-II) 2.21 (2.56) 5.22 (6.19) 18.43 (12.18) Smoking status (% 10.6% 55.1% 92.9% smoker) Average daily cigarettes 2.47 (4.79) 5.08 (7.82) 15.92 (13.02) Alcohol use (AUDIT 3.32 (2.28) 3.86 (4.50) 12.51 (11.53) score) Drug Use DAST-20 0.00 (0.00) 0.51 (1.10) OCDUS 23.86 (9.07) Age onset stimulant use 16.45 (2.86) Years stimulant use 15.74 (6.44) Urine stimulants (%) 92.9% Last stimulant use (days) 2.17 (2.41) Bonferroni group post-hoc comparisons Control vs. Control vs. SDIs vs. Demographics F/χ2 P SDI Sibling Sibling Age 0.68 0.506 0.828 1.00 1.00 Sex (% male) 21.45 <0.001 <0.001 0.205 <0.001 IQ (NART) 2.42 0.093 0.770 0.089 1.00 Education (years) 3.22 0.043 0.039 0.406 0.837 Depression (BDI-II) 53.45 <0.001 <0.001 0.183 <0.001 Smoking status 68.16 <0.001 <0.001 <0.001 <0.001 (% smoker) AUDIT 23.53 <0.001 <0.001 1.00 <0.001 SDI: Stimulant-dependent individuals; NART: National Adult Reading Test; BDI-II: Beck Depression Inventory; AUDIT: Alcohol-Use Disorder Identification Test; DAST20: Drug Abuse Screening Test; OCDUS: Obsessive Compulsive Drug Use Scale. 45 Chapter 2. Cognitive control endophenotype in drug dependence 2.2 Measures The Stroop task was administered during fMRI scanning. Both the colour-word and drugword Stroop (discussed in Chapter 3) were administered in succession during the scanning session, counter-balanced for the order of the two tasks. The tasks were presented in a block design in an attempt to prevent any spill over effects of activation incurred from the salient drug words to the colour-word trials. In the current task, participants were presented with one of four colour words displayed in one of the four font colours – red, blue, green, yellow. They were asked to identify the font colour of the word using a four-button box, each button corresponding to a colour. Participants were trained on button-colour allocation before entering the scanner. During the congruent condition, the word was identical to the font colour in which it was presented; for the incongruent condition the word was displayed in one of the other three colours. Performance was measured by accuracy and response latencies. Interference scores were calculated by subtracting the difference in median RTs on correct trials between the challenge and control conditions (incongruent–congruent). 2.3 Procedure The task was administered as a blocked paradigm to prevent interfering carry-over effects between trial conditions (Ersche, Bullmore, et al., 2010; Nestor et al., 2011). Two blocks were presented containing 16 trials of either congruent or incongruent colour words; the order of words within each block was randomized and the order of blocks was counterbalanced across participants. Each trial lasted 2.2 seconds, the stimulus word presented for 1.9 seconds followed by an inter-trial fixation cross for 0.3 seconds. The allocation of font colours to words was randomized; each of the four colours occurred equally often in each condition and no two identical colours ever followed one another. A 46 Chapter 2. Cognitive control endophenotype in drug dependence cigarette break was allowed no less than one hour before scan time to prevent either nicotine withdrawal or acute nicotine effects from influencing performance. 2.4 Imaging Acquisition Identical imaging acquisition methods were used for all fMRI studies included in this dissertation. Thus, imaging procedures will be described in full detail here, and the reader will be referred to this section for imaging parameters in subsequent chapters. Whole-brain fMRI data were acquired at the Wolfson Brain Imaging Centre, University of Cambridge, Cambridge, England, using a Siemens Magnetom TIM Trio scanner operating 3T (Siemens Medical Solutions, Erlangen, Germany). During the task, 32 transaxial sections of gradient echo, echoplanar imaging (EPI) data depicting blood oxygen level dependent (BOLD) contrast were acquired parallel to the intercommissural line with the following parameters: repetition time (TR)=2000 ms, echo time (TE)=30 ms, flip angle=78°, slice thickness=3 mm plus 0.75 mm, matrix of 64x64 with field of view (FOV)=192x192 mm giving 3x3 mm in-plane resolution. Prior to data analysis, the first five images were discarded for T1 equilibration. T1 structural scans were collected using magnetization-prepared rapid acquisition gradient-echo (MPRAGE) sequence: 176 slices of 1 mm thickness, with TR=2300 ms, TE=2.98 ms, inversion time (TI)=900 ms, flip angle=9°, FOV=240x256 mm. 2.5 Data Analysis Behavioural data were analysed using Statistical Package for Social Science (SPSS v.18; IBM SPSS Statistics, Chicago, Illinois). Analysis of variance (ANOVA) and chi-square tests assessed differences in demographic information. A general linear model (GLM) multivariate analysis in a 2x3 condition by group design with Bonferroni post-hoc corrections was used to compare RTs, as well as interference scores and error rates 47 Chapter 2. Cognitive control endophenotype in drug dependence between groups. Paired samples t-tests assessed RTs within groups. Median scores on correct trials only were used in response latency analyses, and significance levels were set at p<0.05. In preparation for parametric analyses, BDI-II data were square-root transformed to reduce skew. As gender, education, smoking status, AUDIT and BDI-II scores differed between participant groups, multivariate analyses were conducted both with and without these variables as covariates (see Appendix A, Table S2.1). FMRI analysis was conducted using Cambridge Brain Analysis software (CamBA) (http://www-bmu.psychiatry.cam.ac.uk/software/; Cambridge, UK). Data were preprocessed to correct for motion, differential slice-timing and smoothed in plane by 0.5 voxels (Bullmore et al., 1999, 2001; Suckling et al., 2006). Cluster significance levels were set for all imaging analyses using family-wise error (FWE) correction for multiple comparisons p<0.05. Significant cluster values from CamBA fMRI contrast analyses were exported and further evaluated comparing group activation means using GLM multivariate analyses with Bonferroni corrections in SPSS. Correlations between behavioural data and group fMRI contrast activations were also conducted using Pearson correlations in SPSS. First-level whole brain analysis measured activation among all participants contrasting incongruent–congruent conditions on successful trials. A design matrix composed of trial onset and response times was convolved with hemodynamic response function (Glover, 1999), producing statistical maps of voxelwise responses. These maps were normalized to Montreal Neurological Institute (MNI) standard space by affine transformation to an echo-planar imaging template (http://www.fil.ion.ucl.ac.uk/spm) to obtain a group activation map of the contrast. 48 Chapter 2. Cognitive control endophenotype in drug dependence A three-way GLM omnibus analysis in CamBA assessed group differences in activation on the incongruent–congruent contrast. In accordance with previous studies with the Stroop (Bolla et al., 2004; Brewer et al., 2008; Ersche, Bullmore, et al., 2010), as well as our a priori hypothesis, this group analysis was repeated with restricted search volume masks of the IFG and ACC, taken from Hammer’s probabilistic atlas (Hammers et al., 2003). Behavioural interference scores were regressed onto first-level contrast clusters, and within-groups GLM was processed amongst all participants in CamBA. This resulted in a group activation map of significant clusters from the incongruent–congruent contrast that directly correlated with behavioural interference scores in all participants. This analysis was repeated with restriction to the IFG. A grey matter voxel-based morphometry (VBM) analysis previously conducted in these individuals (Ersche, Jones, et al., 2012) was employed with post-hoc application of the IFG mask to focus differences in cortical volume to this region. The VBM analysis was conducted by P. S. Jones using FSL (http://www.fmrib.ox.ac.uk/fsl/fslvbm/index.html, Oxford, UK) on T1-weighted images collected during the same session as the functional data, and then compared for group differences using CamBA software for permutation testing (Smith & Nichols, 2009). In the current study, IFG grey matter volume was compared between groups using ANOVA and correlated with behavioural performance using Pearson coefficients. 3. Results Stimulant-dependent individuals had been on drugs for an average of 15.7 years (±6.4 SD), beginning use at the age of 16.5 (±2.9 SD). Ninety-three percent (n=39) tested 49 Chapter 2. Cognitive control endophenotype in drug dependence positive for stimulants at the time of testing using urinalysis drug screen, with a mean time since last use of 2.2 days (±2.4 SD). SDIs had significantly higher depression and alcohol abuse scores than both sibling and control participants. There were also significantly more males in the stimulant-dependent group and higher rates of cigarette smoking. Control and sibling participants did not differ in terms of sex, depression rates or alcohol use, though there were differences between the two groups in smoking status (Table 2.1). There were no differences in age or IQ between any of the three groups, and only SDIs and controls differed in terms of educational attainment. 3.1 Stroop Performance Using GLM multivariate analyses, significant differences arose between groups on response latencies for congruent (F(2,135)=7.243, p=0.001) and incongruent (F(2,135)=4.452, p=0.013) trials (Table 2.2). These values remained significant after controlling for gender, education, smoking status, alcohol use and BDI-II depression scores in the model. As there were no differences in any of the results when using covariates or not, we report the rest of the results without covariates in the model. See Supplemental Information (Appendix A, Table S2.1) for a full set of results with covariates. In the congruent condition SDIs had significantly slower responses than controls (Bonferroni post-hoc p=0.001), while the siblings were slower than controls at p<0.10. On incongruent trials, both the SDIs (p=0.031) and their siblings (p=0.035) were significantly slower than controls. Stimulant-dependent and sibling groups did not differ from one another in either condition. There were no differences between groups on interference scores contrasting congruent from incongruent trials (F(2,135)=0.797, p=0.45). However, in a paired samples t-test all three groups were significantly slower on the incongruent than congruent condition 50 Chapter 2. Cognitive control endophenotype in drug dependence within their own cohorts (controls: t(46)=7.319, p<0.001; SDI: t(41)=5.372, p<0.001; siblings: t(48)=7.327, p<0.001). This confirms the additional cognitive load of the incongruent trials, regardless of group. Groups did not differ in the number of errors made on the task (F(2,135)=1.915, p=0.151). Table 2.2. Behavioural results and group comparisons on the colour-word Stroop Control Mean (SD) Sibling Mean (SD) SDI Mean (SD) F/χ2 P Congruent med RT 653.17(125.99) 723.14(137.50) 768.99(171.36) 7.24 0.001 Incongruent med RT 802.72(181.25) 904.85(214.90) 910.76(188.10) 4.45 0.013 Interference med RT 149.55(140.08) 181.70(173.58) 141.77(171.05) 0.80 0.454 2.30 (7.2%) 3.37 (10.5%) 3.43 (10.7%) 1.92 0.151 Behavioural Results Total mean errors Congruent and incongruent trial results represented via median response latencies (ms); interference scores reported as the difference in response times between the two trial conditions (ms); total mean errors and error percentage are given. 3.2 Neuroimaging Analysis In a first-level analysis, four clusters emerged that significantly differed in BOLD activation on the incongruent–congruent contrast among all participants. Increases in activity on incongruent compared with congruent trials were seen in the left IFG, stretching into the dorsolateral prefrontal cortex and precentral/middle frontal gyrus, while decreases in activation on the incongruent compared with congruent trials were present in the right rolandic operculum and caudate (Figure 2.1, Table 2.3). These differences in BOLD signal represent greater (or less) relative activation in all participants during performance of incongruent word trials, as compared to activity present during performance of congruent word trials. Follow-up analyses using a GLM 51 Chapter 2. Cognitive control endophenotype in drug dependence multivariate analysis conducted in SPSS comparing group activation in these four clusters did not reveal any differences in activity levels between groups. A) X= -46 Y=6 Z=38 X=50 Y= -24 Z=12 B) Figure 2.1. Whole-brain fMRI activation among stimulant-dependent individuals, their non-dependent siblings and healthy control volunteers on the Stroop, contrasting congruent from incongruent colour word median response latencies. Four clusters were identified as having significantly different activation on incongruent than congruent conditions, with peak values in: A) the left precentral gyrus (Montreal Neurological Institute coordinates (x, y, z): -44, 4, 34) and left inferior frontal gyrus (-50, 14, 28); and B) the right caudate (4, 4, 12) and right rolandic operculum (60, -22, 12). Activation in these regions did not differ between groups. 52 Chapter 2. Cognitive control endophenotype in drug dependence Table 2.3. Mean activation cluster voxels for imaging contrasts amongst all groups Brodmann Cluster size Peak value Contrast activation areas Area (voxels) coordinates Whole-brain contrast (incongruent-congruent) analysis among all participants Cluster 1 Right rolandic operculum, right superior temporal 13, 22, 40, 105 60, -22, 12 gyrus, right heschl gyrus, right insula, right 41, 42, 43 postcentral gyrus, right supramarginal gyrus Cluster 2 Right caudate 125 59 4, 4, 12 Cluster 3 Left inferior frontal gyrus – triangularis, 9, 45, 46 114 -50, 14, 28 opercularis, left precentral gyrus Cluster 4 Left precentral gyrus, left middle frontal gyrus, 6, 8, 9 126 -44, 4, 34 left postcentral gyrus Between group contrast comparison Cluster 1 Right rolandic operculum, right insula, right 13 232 40, -26, 28 caudate, right supramarginal gyrus Cluster 2 Left medial superior frontal gyrus, left middle 8, 24, 32 110 -28, 14, 34 frontal gyrus, left superior frontal gyrus, left inferior frontal gyrus – opercularis Between group comparison with IFG Mask Cluster 1 Left inferior frontal gyrus – opercularis, 13 43 -42, 10, 20 triangularis, left rolandic operculum, left insula Interference regression activation with IFG Mask Cluster 1 Left inferior frontal gyrus – triangularis, left 13, 45, 47 25 -38, 28, 4 insula Cluster 2 Right inferior frontal gyrus – triangularis, 13, 45 30 42, 24, 12 operculum Contrasts represent activation on incongruent compared to congruent trials, first amongst all participants and then comparing contrast activations between groups. Interference scores are regressed onto contrast activations in the fourth analysis, using all participants. Significance set a p<0.05 family-wise error correction for multiple comparisons. Coordinates listed are in MNI standard space. 53 Chapter 2. Cognitive control endophenotype in drug dependence In a whole-brain omnibus group comparison, two clusters emerged that significantly differed between groups during the incongruent–congruent contrast: right insula/rolandic operculum (F(2,135)=18.373, p<0.001) and left medial/superior frontal gyrus (F(2,135)=12.094, p<0.001; Table 2.3). In these regions, siblings registered a significant relative decrease in activation compared to control and stimulant-dependent participants (Bonferroni p<0.001 for all comparisons). SDIs and controls did not differ from one another. After restriction of the analysis to the IFG, an additional cluster was revealed in the left hemisphere that significantly differed between groups during the incongruent–congruent contrast (F(2,135)=11.981, p<0.001). The siblings again had significantly lower activity than both SDIs (p<0.001) and controls (p=0.006), whereas SDIs and controls did not significantly differ (Figure 2.2, Table 2.3). Application of a mask of the ACC did not result in any significant clusters emerging that differed in activation in this region between the three groups. 54 Chapter 2. Cognitive control endophenotype in drug dependence A) SDIs B) P A R LR Y=10 X= -38 L Z=20 Figure 2.2. Activation differences in the IFG between groups during Stroop performance, with application of an IFG mask. Siblings demonstrated a significant decrease in activation in the left IFG compared with both SDIs and controls, whereas SDIs and controls did not differ from one another in this region. A) Group differences in fMRI activation in the left IFG, representing a decrease in activation in the siblings. B) Left IFG cluster. 55 Chapter 2. Cognitive control endophenotype in drug dependence 3.3 Regression of Stroop Performance to Neuroimaging Findings When behavioural interference scores were regressed onto the incongruent–congruent imaging contrast in CamBA no significant areas were found. However, upon application of the IFG mask two bilateral clusters arose that were significantly deactivated in association with interference scores among all participants. Follow-up analyses in SPSS confirmed that activity in these areas negatively correlated with behavioural performance in all participants (left: r=-0.351, p<0.001; right: r=0-.398, p<0.001), with greater deactivation during the contrast signifying greater interference. When each group was analysed individually, the correlation with interference scores during the incongruent–congruent interference regression contrast remained significant in the left (r=-0.454, p=0.001) but not right IFG for control participants. Among SDIs, interference scores negatively correlated with activity in both the left and right IFG during the incongruent–congruent interference regression contrast (left: r=-0.366, p=0.017; right: r=-0.580, p<0.001). In sibling participants only right IFG activation in the incongruent–congruent interference regression contrast negatively correlated with interference scores (r=-0.377, p=0.008). 3.4 Relationship of Structural Changes to Stroop Performance A structural analysis comparing grey matter volume between these groups revealed further differences in performance based on cortical volume. Upon application of the IFG mask onto the VBM analysis, we discovered differences in grey matter volume bilaterally in the IFG in both SDIs and their siblings as compared to controls (left: F(2,135)=11.749, p<0.001; right: F(2,135)=9.384, p<0.001). These structural changes related to behavioural performance, such that bilateral decreases in IFG grey matter volume were correlated with RTs on both congruent and incongruent trials among all participants (congruent: left 56 Chapter 2. Cognitive control endophenotype in drug dependence r=-0.337, p<0.001; right r=-0.320, p<0.001; incongruent: left: r=-0.253, p=0.003; right r=-0.224, p=0.008; Figure 2.3). However, interference scores did not correlate with cortical volume. Additionally, IFG volume and BOLD activity levels did not correlate. A) Differences in IFG Grey Matter Volume Between Groups L inferior frontal gyrus Mean Grey Matter Volume R inferior frontal gyrus SDIs B) Correlation Between Response Times and IFG Grey Matter L inferior frontal gyrus Mean Grey Matter Volume R inferior frontal gyrus Mean Response Time 57 Chapter 2. Cognitive control endophenotype in drug dependence Figure 2.3. Structural differences in the IFG between stimulant-dependent individuals (SDIs), their siblings and control participants. A) SDIs and siblings both demonstrated a significant decrease in grey matter volume in the bilateral IFG as compared to controls. There was no difference in volume between SDIs and their siblings. B) IFG grey matter volume decreases significantly correlated bilaterally with an increase in response times on both conditions of the colour-word Stroop task, with a decrease in volume relating to an increase in response latencies. 3.5 Effects of Drug Use History on Performance Among the SDIs, there was a significant correlation between years of stimulant use and errors made on the task (r=0.388, p=0.011), such that the longer an individual had used stimulant drugs, the greater number of errors were committed. There were no other significant relationships between drug use history and either colour-word Stroop performance or neuroimaging activation. This includes time since last stimulant use, which did not correlate with any measure of task ability, including reaction times, interference scores and errors committed, or functional activation on the Stroop. A median split for time since last use (≥ 2 days) also did not reveal any significant differences in either behavioural or functional performance. 4. Discussion Stimulant-dependent individuals and their non-dependent biological siblings were significantly more impaired on the colour-word Stroop task than unrelated healthy control volunteers, as demonstrated by longer response latencies during the task. However, the sibling-pairs did not differ from one another on any variable, and there were no differences between any of the groups in interference scores. In terms of BOLD activity, 58 Chapter 2. Cognitive control endophenotype in drug dependence significant differences were evident between the sibling participants and both stimulantdependent and control individuals, with the siblings comparatively under-activating the bilateral IFG and left superior/middle frontal gyrus. However, the SDIs and controls did not differ in activity levels in any region. Two primary questions arise from these results: why was there no difference in activity between SDIs and controls despite differences in behavioural performance?; and why did the siblings significantly under-activate frontal gyral regions compared with the other groups? The lack of differentiation between groups on interference scores suggests that, despite slowing in the SDIs and siblings, there was no greater dysfunction on the challenging incongruent condition. Instead, there appeared to be globalised slowing or inefficiency rather than task-specific impairment. This absence of differences in interference scores was not unexpected, as previous studies investigating colour-word Stroop performance in SDIs have shown no impairments in behavioural performance compared with control individuals (Barros-Loscertales et al., 2011; Bolla et al., 2004; Ersche, Bullmore, et al., 2010; Goldstein et al., 2001). Cognitive slowing corresponded to structural differences in the IFG in both SDIs and their siblings, with decreases in grey matter volume correlating with increased response times on both conditions. This suggests that these structural changes affected cognitive efficiency, but did not cause a condition-specific impairment. Earlier findings in these groups support these results, with decreases in white matter connectivity adjacent to the IFG associated with increased slowing on a motor control task (Ersche, Jones, et al., 2012). The question remains as to why SDIs, despite their similar patterns of activation, had such a significant increase in activity compared with their siblings. It is possible that the hypoactivation exhibited by the siblings was the endophenotype-like response, and that SDIs 59 Chapter 2. Cognitive control endophenotype in drug dependence prior to drug abuse would have demonstrated similar decreases in activation during inhibitory control. However, the effect of stimulant use, whether chronic or acute, may have altered this original response. An assessment of motor inhibition in adolescents showed similar results, with a relative over-activation in the same right IFG region in adolescents who had experimented with drugs compared with those who had not (Whelan et al., 2012). This suggests that the increase in neural activation during cognitive control (including inhibitory response) occurs relatively early as a consequence of drug abuse. Conversely, the decreased activation in the siblings could be representative of a protective factor against drug abuse, despite similar manifestations of behavioural impairments in inhibitory control. This account suggests that SDIs and siblings may experience similar baseline activation, but that the effects of stimulants could have elevated stimulant users’ BOLD activity to that of controls. Alternatively, as 93% of SDIs tested positive for stimulants, their surprising relative hyper-activation compared with their siblings could be an acute effect of stimulant drugs on the brain. Cocaine can increase neural activity (Breiter et al., 1997), elevating low baseline levels in abstinent users to those of non-drug-using controls (Garavan, Kaufman, & Hester, 2008). This may explain the lack of difference in BOLD activation between SDIs and control participants in the current study, which is in conflict with previous investigations of the colour-word Stroop in dependent drug users (Barrós-Loscertales et al., 2011; Bolla et al., 2004; Brewer et al., 2008; Nestor et al., 2011). This phenomenon has also been proposed to support the self-medication hypothesis in individuals with decreased dopaminergic activity. However, in the current investigation time since last use did not correlate with any behavioural or functional imaging results in the SDIs. Moreover, a median split conducted on time since last use did not reveal any significant differences in performance or activation between individuals with shorter or longer 60 Chapter 2. Cognitive control endophenotype in drug dependence periods of abstinence. Thus, we do not believe that either the acute effect of stimulants or stimulant withdrawal significantly affected SDIs’ behavioural performance or functional activation on the task. Finally, as the colour-word Stroop task was administered in succession with the cocaineword Stroop task, it is possible that participants who completed the cocaine-word Stroop task first could have experienced a carry-over effect of heightened BOLD activation that was initially in response to the salient cocaine cue words. This may have resulted in an unintentional increase in activation during subsequent non-salient trials. However, the task was administered as a counter-balanced block design in an attempt to prevent this spill over from occurring, and only half of the SDIs would have completed the task in this order. Thus, we do not believe that the results in the current study were significantly affected by this task design. The absence of activation in the ACC, a key area in Stroop performance (Bolla et al., 2004; Brewer et al., 2008; Kerns et al., 2004; MacDonald et al., 2000; Salo et al., 2002) was perhaps surprising. However, absence of cingulate activation on the Stroop has also been noted in other studies with drug using participants (Ersche, Bullmore, et al., 2010; Zysset et al., 2001). The ACC is thought to control conflict monitoring, and it is most activated during changing task demands (such as switching from congruent to incongruent stimuli), resulting in greater cognitive conflict (Kerns et al., 2004). However, the present study used a block design, circumventing the change in task demands. This adjustment of task structure may explain the lack of ACC activation, as there was no conflict between task demands within each block. Additionally, greater ACC activation on error trials is commonly cited, with incorrect responses requiring behavioural adjustments on subsequent trials (Kerns et al., 2004). As only correct responses were 61 Chapter 2. Cognitive control endophenotype in drug dependence included in the current model, it is possible this region was not significantly recruited during correct responding. Finally, dissociation between IFG and ACC activation during Stroop performance has been suggested, the ACC compensating for diminished IFG control (MacDonald et al., 2000). Given the significant increase in IFG activity, particularly in SDIs and controls, it is possible that the ACC was not requisitely recruited by participants during the current task. To further explore the different effects of acute and chronic stimulant use, as well as the presence or absence of an underlying risk for dependence, research into an alternative group of cocaine users – individuals who use the drug recreationally without developing dependence – will be discussed in the next chapter to help parse out the impact of these different variables. Additionally, the colour-word Stroop task is a measure of ‘cold’ cognitive function, assessing inhibitory control in a neutral valence condition. However, as stimulant-dependent individuals are known to also have impairments in their responses to more salient stimuli, particularly in regard to drug-related cues, we explored differences in inhibitory processes in the face of emotional stimuli in the next chapter using a drug-word Stroop task. 62 Chapter 3. Absence of cocaine cue bias in recreational stimulant users Chapter 3. Enhanced Orbitofrontal Cortex Function and Lack of Attentional Bias to Cocaine Cues in Recreational Stimulant Users 1. Introduction In addition to the cognitive dysfunction present in dependent stimulant users discussed in the previous chapter, there is profound evidence of a disruption in affective system processing, thought to stem from abnormalities in the frontostriatal reward circuitry. This is particularly evident in the face of salient drug stimuli, where the associated cues are thought to ‘hijack’ the reward system, emphasizing drug rewards over other priorities. This can lead to significant drug craving, which can in turn cause unplanned or undesired use. Attentional bias to drug-related cues can elicit feelings of craving and, combined with the poor decision-making and inhibitory control characteristic of stimulant-dependent individuals (SDIs), can precipitate relapse (Bechara, 2005; Copersino et al., 2004; Field & Cox, 2008; Garavan et al., 2000). These experiences are thought to be subserved by dysfunction in the prefrontal cortex (PFC) (Everitt et al., 2007; London et al., 2000; Schoenbaum & Shaham, 2008), where, as discussed previously, dependent stimulant users have been shown to have decreased grey matter volume compared with healthy control individuals (Alia-Klein et al., 2011; Ersche et al., 2011; Franklin et al., 2002; Hanlon, Dufault, Wesley, & Porrino, 2011; Sim et al., 2007). Additionally, SDIs typically exhibit a significant decrease in PFC activation on executive function tasks, often accompanied by behavioural impairments in self-control, inhibition and working memory (Barros-Loscertales et al., 2011; Bolla et al., 2004; Nestor et al., 2011; Tomasi et al., 2007; Verdejo-Garcia et al., 2006). 63 Chapter 3. Absence of cocaine cue bias in recreational stimulant users However, it is important to note that although these cognitive impairments can be serious, they do not afflict all drug users. In fact, the vast majority of individuals who try stimulant drugs do not become addicted to them (United Nations Office on Drugs and Crime, 2012). Moreover, there seems to be a select subset of the population who are able to use cocaine recreationally in a controlled manner without developing dependence (Ersche, Jones, Williams, Smith, et al., 2013). These individuals report consistent, occasional, social use of cocaine without experiencing a loss of control or exhibiting symptoms of dependence or abuse (Ersche, Jones, Williams, Smith, et al., 2013). They also do not report feeling cravings for cocaine, and their use is planned rather than impulsive. These individuals who have used cocaine in a stable manner for an extended period of time without developing a dependency are an intermediary group that can be used to assess potential cocaine-induced abnormalities and distinguish them from traits involved in compulsive use and dependence. Recreational users would be expected to show similar, though not as severe, changes in structure and function attributed to prolonged stimulant use, but not the abnormalities associated with increased premorbid risk for dependence. Furthermore, there may be additional differences between the brains of dependent and recreational stimulant users that serve as protective factors in these nondependent individuals (Ersche, Jones, Williams, Smith, et al., 2013). However, it should be noted that inherent differences in cocaine exposure between the dependent and recreational users may create potential confounds when comparing cognitive function and attentional bias to drug cues. The emotional cocaine-word Stroop is a well-established measure of cognitive control and attentional bias to cocaine-related stimuli (Field & Cox, 2008). Like the classic 64 Chapter 3. Absence of cocaine cue bias in recreational stimulant users version of the Stroop task, participants are asked to ignore the content of a target word, responding instead to the colour of the font. Performance is assessed via a relative increase in response latencies to cocaine cues compared to those for matched neutral words. Impairment stems from a problem with attention allocation and selective processing of more salient cues, as stimulant users become distracted by the cocaine words (Carpenter et al., 2006; Copersino et al., 2004; Ersche, Bullmore, et al., 2010; Hester et al., 2006). Preoccupation with cocaine stimuli is thought to interfere with the normal cognitive processes required to name the font colour of the word, resulting in higher reaction times than on emotionally neutral words (Cox, Fadardi, & Pothos, 2006; Field & Cox, 2008). In several studies this bias has been linked to cocaine craving scores, with longer response latencies correlating with greater self-report levels of craving (Copersino et al., 2004; Franken, Kroon, & Hendriks, 2000). In the current study, we investigated selective bias to cocaine-cue words on an emotional Stroop task using a functional magnetic resonance imaging (fMRI) paradigm. We assessed the same groups of 50 stimulant-dependent individuals and 50 healthy control volunteers from the previous chapter, with the addition of a new group of participants – 27 recreational users of cocaine – in an attempt to determine the effect of drug use severity on attentional bias to cocaine-related cues. Data was collected from the recreational users after the original study was completed; however, an identical testing battery and study procedures were used in these individuals. There were no differences in the scanning facility or parameters used during the experiment, ensuring consistency between the groups. For the cocaine Stroop task, the sibling individuals from the previous chapter were excluded from the contrast as the current investigation was specifically testing the effects 65 Chapter 3. Absence of cocaine cue bias in recreational stimulant users of cocaine exposure on responses to drug-related cues, not endophenotype effects. This means that the primary contrast was between the SDIs, recreational users of cocaine and unrelated control volunteers. The sibling individuals could not be used as control subjects in the current assessment as they had greater than normal experience with stimulantrelated stimuli via their dependent brothers and sisters (though no direct exposure to stimulant drug-taking themselves), which can still result in salient drug associations (Barnard, 2005). However, as they had no personal direct stimulant exposure they could not provide any insight into mechanisms regarding automatic drug-cue associations, or the resistance to such salient connections. We compared the groups on both their behavioural and neural responses to cocaine versus neutral stimuli during the Stroop. To the best of our knowledge, this is the first investigation into the functional neural activity of recreational cocaine users, as well as the first to assess responses to cocaine-related stimuli in this group. We predicted that recreational users would fall between stimulant-dependent and control individuals in terms of both behavioural and neurocognitive responses, given their greater familiarity with cocaine cues but non-dependent patterns of use. 2. Materials and Methods 2.1 Participants As in the previous chapter, 50 stimulant-dependent individuals were recruited through drug-treatment centres and word-of-mouth; 27 recreational cocaine users and 52 healthy control volunteers were recruited from the community using local advertisements. Two additional control volunteers were added to the sample from the previous study in an attempt to better match the groups, taking into account the new cohort of recreational users. All SDIs met DSM-IV-TR criteria for stimulant dependence; control volunteers 66 Chapter 3. Absence of cocaine cue bias in recreational stimulant users had no personal or family history of drug abuse or dependence. Recreational users had been using cocaine for a minimum of two years without displaying any DSM-IV-TR symptoms of physical or psychological dependence. Cocaine non-dependence was screened for in these individuals focusing not only on frequency and amount of use, but also on patterns of use and associated cognitive and emotional perceptions. For example, recreational participants reported no feelings of guilt or remorse about using and stated that their occasional use did not interfere with work, school, family or social obligations. They also reported no feelings of cravings or urges to use the drug and could “take it or leave it”. The Obsessive Compulsive Drug Use Scale (OCDUS) was also administered to assess drug use severity, measuring thoughts and obsessions regarding cocaine use, as well as success at resisting these urges (Franken et al., 2002). Drug urinalysis was collected from all participants, and control and recreational users tested negative for all substances. Conversely, 93% of SDIs tested positive for stimulants. Members from all three groups also reported either current or prior use of tobacco and cannabis. As in the previous chapter, depression scores, alcohol use tendencies and verbal IQ were assessed using the Beck Depression Inventory (BDI-II) (Beck et al., 1988), Alcohol-Use Disorder Identification Test (AUDIT) (Saunders et al., 1993), and National Adult Reading Test (NART) (Nelson, 1982), respectively. In the present task, 15 individuals were excluded from analysis, resulting in a total of 114 participants. Reasons for exclusion included incomplete dataset, excessive head motion during scanning, poor understanding of task requirements, and neurological abnormalities discovered post-hoc. An updated table with demographic information for the present study is included below (Table 3.1). 67 Chapter 3. Absence of cocaine cue bias in recreational stimulant users Table 3.1. Demographic information and group differences for 41 dependent and 26 recreational stimulant users, and 47 healthy control volunteers Control Mean (SD) Recreational Mean (SD) Dependent Mean (SD) F/χ2 P 32.52 (8.86) 33 (63.5%) 112.61 (8.18) 12.69 (1.92)a 2.27 (2.54)a 3.35 (2.25)a 30 (57.7%)ab 11 (21.2%)ab 29.15 (7.56)c 14 (51.9%)c 115.64 (5.39)c 13.41 (1.74)c 3.78 (4.32)c 5.74 (0.30)c 23 (88.9%)bc 26 (96.3%)b 34.28 (7.25)c 44 (88.0%)c 110.64 (7.52)c 11.58 (1.74)ac 18.08 (11.91)ac 11.14 (1.59)ac 49 (98.0%)ac 50 (100%)a 3.609 13.127 3.609 9.917 58.405 15.703 23.640 85.116 0.030 0.001 0.030 <0.001 <0.001 <0.001 <0.001 <0.001 100.35 (96.86) 21.26 (5.10) 7.85 (5.87) 1.19 (1.62) 11 (40.7%) 2.78 (4.70) 19.56 (4.81) 12.64 (6.51) 23.66 (9.35) 33 (66.0%) -7.08 -1.44 3.145 12.35 4.57 <0.001 0.155 0.002 <0.001 0.033 Demographics Age* Gender (n male:%)* NART (score)* Education (years)* BDI-II (total score)* AUDIT (total score)* Tobacco use history (%)* Cannabis use history (%)* Drug Use Last stimulant use (days)* Age of use onset (years) Duration of use (years)* OCDUS (total score)* Cannabis use (n:%)* *Significant group difference at p<0.05. a Significant difference between Dependent and Control groups at Bonferroni post-hoc p<0.01. Recreational and Control. c b Significance between Significance between Dependent and Recreational. NART: National Adult Reading Test; BDI-II: Beck Depression Inventory; AUDIT: Alcohol-Use Disorder Identification Test; OCDUS: Obsessive Compulsive Drug Use Scale. 2.2 Behavioural Measures All participants completed the cocaine-word Stroop task during fMRI scanning. The task consisted of two 16-trial blocks of either common cocaine-related words (i.e. coke, line, snort) or neutral words that were matched for length and frequency of use (i.e. song, band, piano). Each trial lasted 2.2 seconds, the target word presented for 1.9 seconds 68 Chapter 3. Absence of cocaine cue bias in recreational stimulant users followed by an inter-trial interval of 0.3 seconds. Word order was randomised within each block and blocks were counter-balanced between participants. Individuals were asked to respond to the font colour of the word using a four-button box, with each button corresponding to one of the four font colours (red, blue, green, yellow). Participants were trained on the task and button-colour allocation before entering the scanner. Performance was measured using response latencies and accuracy. Interference scores were calculated as the difference between response times on cocaine and neutral trials. 2.3 Behavioural Analysis As before, data were analysed using the Statistical Package for Social Science (SPSS v.18). Demographic information and error scores were analysed using analysis of variance (ANOVA), chi-square and independent samples t-tests. Response times and interference scores were compared between groups using general linear model (GLM) multivariate and repeated-measures analyses, controlling for age, gender, education, smoking status, BDI-II and AUDIT scores. Post-hoc analyses were conducted using Bonferroni tests. Pearson correlation coefficients were used to assess relations between variables, and paired samples t-tests compared categorical differences in response times within groups. Median values were used for response latency analyses and only correct trials were included; significance levels were set at p<0.05. 2.4 Imaging Analysis Imaging acquisition procedures were identical to those discussed in the previous chapter; see Chapter 2, Section 2.4 for detailed information. FMRI data were analysed using Cambridge Brain Analysis software (CamBA) (http://www-bmu.psychiatry.cam.ac.uk/software/; Cambridge, UK). Images were motioncorrected and registered to Montreal Neurological Institute (MNI) standard stereotactic 69 Chapter 3. Absence of cocaine cue bias in recreational stimulant users space using affine transformation (http://www.fil.ion.ucl.ac.uk/spm), and were spatially smoothed in-plane with a Gaussian kernel of 0.5 voxels FWHM. The presentation time of each word was modelled as an event onset, with the duration as time until response. Each event was convolved with a canonical blood oxygen level dependent (BOLD) hemodynamic response function in CamBA to assess brain activation up to the time of response on all trials. A comparison of BOLD responses during cocaine versus neutral word trials was conducted, first among all participants and then comparing contrast activation between groups. Analyses were repeated using the inferior frontal gyrus (IFG) as a region of interest, given our significant findings from the previous chapter, as well as reports from other studies identifying the IFG as a region involved in Stroop task performance (Ersche, Bullmore, et al., 2010) and an area commonly found to be abnormal in stimulant-dependent individuals (Bolla et al., 2004). Significance levels were set at family-wise error (FWE) correction p<0.05 for all analyses. Whole-brain imaging analysis compared activation during cocaine and neutral word conditions on successful trials among all participants, creating a group contrast map of significant voxel-wise responses of the cocaine–neutral contrast. Significant clusters were then exported to SPSS and compared between groups using GLM multivariate analysis and Bonferroni post-hoc corrections, including the demographic variables listed above as covariates in the model. Between-group activation differences were also assessed using a three-way GLM omnibus analysis in CamBA, identifying clusters that significantly differed between groups on the cocaine versus neutral contrast. These results were then exported into SPSS for further analysis, comparing group differences in BOLD response in the identified clusters using GLM multivariate analysis. 70 Chapter 3. Absence of cocaine cue bias in recreational stimulant users A within-group GLM was conducted in CamBA, regressing behavioural interference scores onto the group activation contrast map. This identified regions in which BOLD contrast activity levels directly correlated with cocaine interference scores. These regions were further analysed in SPSS using Pearson correlation and GLM multivariate analyses. Within-group GLM and between-group GLM omnibus analyses were repeated after application of a restricted small volume mask of the IFG taken from Hammer’s probabilistic atlas (Hammers et al., 2003). 3. Results The three groups significantly differed in terms of age and gender, with recreational users being younger than stimulant-dependent individuals and more females present in the control and recreational groups. SDIs also had significantly lower verbal IQ scores than recreational users and fewer years of education than both control and recreational participants. There were higher rates of tobacco and cannabis use in the stimulantdependent and recreational users, as well as higher alcohol and depression scores in SDIs. As such, age, gender, education, smoking status, BDI-II and AUDIT scores were controlled for in all analyses. 3.1 Behavioural Performance Stimulant-dependent individuals were more impaired than both recreational and control participants on the cocaine-word Stroop task, demonstrated via a greater number of errors committed on both cocaine (Kruskal-Wallis χ2=23.01, p<0.001) and neutral trials (χ2=6.62, p<0.036), and increased interference scores (F(2,105)=3.017, p=0.053), though this lost significance after controlling for demographic variables. Conversely, recreational users did not differ from controls on any behavioural measure (see Appendix B, Table S3.1 for full behavioural results). In a mixed-model ANOVA with participant group and 71 Chapter 3. Absence of cocaine cue bias in recreational stimulant users word type (cocaine versus neutral), SDIs had significantly slower response times compared with the other two groups (F(2,105)=3.787, p=0.026). Within the groups, SDIs also demonstrated attentional bias to cocaine cues, with significantly increased response latencies to cocaine over neutral stimuli (t(40)=3.665, p=0.001). Neither recreational users nor controls had an increase in response times from cocaine to neutral words (control: t(46)=1.238, p=0.222; recreational: t(25)=-1.561, p=0.131). 3.2 Functional Imaging Six clusters emerged that demonstrated significantly different activation during cocaine versus neutral trials amongst all participants (Figure 3.1; Appendix B, Table S3.2). This included increases in left orbitofrontal and inferior frontal gyrus activity, as well as bilaterally in the superior medial frontal lobe and anterior cingulate cortex. A significant decrease in activation was also seen in the right cuneus/precuneus and superior and inferior parietal lobe. Activity in these regions did not differ between groups. 72 Chapter 3. Absence of cocaine cue bias in recreational stimulant users A) X=2 Y=5 0 Z= -20 X= -46 Y=26 0 Z= -8 B) Figure 3.1. Significant clusters identified during the first-level fMRI contrast, comparing activation in response to cocaine versus neutral words amongst all participants. Six significant clusters were identified; regions shown include increases in activation on cocaine compared to neutral trials in the A) left orbitofrontal cortex, bilateral superior medial frontal gyrus and bilateral anterior cingulate cortex, and B) left inferior frontal gyrus and left angular gyrus. Activation in these areas did not differ between participant groups. Significance was set at p<0.05 family-wise error correction for multiple comparisons. Coordinates listed are in MNI standard space. Two clusters in the right orbitofrontal/anterior cingulate cortex and right angular gyrus/posterior cingulate cortex significantly differed between groups during a secondlevel GLM omnibus contrast comparing activation to cocaine–neutral trials between participants. In both of these areas, recreational users demonstrated a significant decrease in activity compared with SDIs and control participants. There were no differences in activation levels between controls and SDIs in these regions (Figure 3.2; Appendix B, Table S3.3). 73 Chapter 3. Absence of cocaine cue bias in recreational stimulant users A) Cluster 1 – R OFC/ACC Cluster 2 – R angular gyrus B) X=16 Y=48 Z= -4 Figure 3.2. Second-level group contrast, comparing activation between groups during the cocaine–neutral word contrast. Significant differences emerged in two clusters: the right orbitofrontal cortex and anterior cingulate cortex, and the right angular gyrus and posterior cingulate cortex. Recreational cocaine users significantly under-activated these two regions in comparison with the other two groups. Coordinates listed are in MNI standard space. Cluster significance set at 74 Chapter 3. Absence of cocaine cue bias in recreational stimulant users p<0.05 family-wise error correction for multiple comparisons. Group comparisons made using ANCOVA models, controlling for age, gender, years of education, smoking status, BDI-II depression and AUDIT alcohol scores, with Bonferroni posthoc correction p<0.05. After restricting analysis to the inferior frontal gyrus, three additional clusters emerged that significantly differed between participants in the cocaine–neutral word GLM omnibus contrast (Appendix B, Table S3.3). These three areas spanned across the right IFG, including the insula and operculum. Control participants showed a significant underactivation in the first cluster compared with both dependent and recreational users; these two groups did not differ from each other in this area. In the second cluster, there was again a significant decrease in activity in the recreational participants compared with both stimulant-dependent and control individuals, whereas in the third cluster the dependent stimulant users had significantly greater activation compared to recreational users but not controls (Figure 3.3). 75 Chapter 3. Absence of cocaine cue bias in recreational stimulant users A) IFG cluster 1 IFG cluster 2 IFG cluster 3 B) X=36 Y=14 Z=10 Figure 3.3. Between-group differences in contrast activation in the lateral inferior frontal gyrus (IFG) upon application of a small-volume correction mask. Stimulantdependent individuals demonstrated an increase in activity throughout the IFG, whereas recreational users showed a relative decrease in activity in two of the three clusters, but had equal levels to dependent users in the third. Control participants 76 Chapter 3. Absence of cocaine cue bias in recreational stimulant users typically fell between the two stimulant using groups in terms of activation. Coordinates listed are in MNI standard space. Cluster significance set at p<0.05 family-wise error correction for multiple comparisons. Group comparisons made using ANCOVA models controlling for age, gender, years of education, smoking status, BDI-II depression and AUDIT alcohol scores, with Bonferroni post-hoc correction p<0.05. When interference scores were regressed against activation levels among all groups on the cocaine–neutral contrast, no significant clusters arose. However, upon application of a mask of the IFG, three areas in the right IFG emerged where activity significantly correlated with cocaine interference scores in all participants. These were centred in the right IFG pars triangularis and pars orbitalis. The trend again remained of recreational users showing a comparative under-activation of these regions, while dependent stimulant users exhibited a relative over-activation (Appendix B, Figure S3.1); however, these differences were only significant in the cluster centred on the right IFG pars orbitalis. Activity levels in control participants fell between the other two groups, and did not differ from either recreational users or controls. Activation in all three regions significantly negatively correlated with interference scores in all groups, with greater impairment associated with greater activity on cocaine versus neutral trials (r=0.377, 0.405, 0.381; p<0.001 all correlations). 4. Discussion Recreational cocaine users were unimpaired on a cognitive control task measuring attentional bias to cocaine-related stimuli, performing on par with non-drug using control participants. Conversely, stimulant-dependent individuals were significantly impaired 77 Chapter 3. Absence of cocaine cue bias in recreational stimulant users compared to the other two groups, registering longer response times, higher interference scores and more errors committed. Recreational users also demonstrated very different patterns of activation during task performance, significantly under-activating prefrontal and orbitofrontal regions compared with both stimulant-dependent and control individuals. The absence of a significant increase in response times among recreational users to cocaine-related stimuli indicates that they do not share an attentional bias to these words with dependent users. Drug-related attentional bias has been linked to increased motivation to obtain the substance, as well as heightened emotional salience for these cues (Field & Cox, 2008; Goldstein & Volkow, 2002). The absence of preferential attention to cocaine cues in the recreational users suggests an inherent difference in automatic drug-related stimulus processing between the two participant groups, underlying an intrinsic difference in the pattern and type of drug use. Similar results have been reported in dependent and recreational cannabis users, with more severe users reporting greater drug craving and increased attentional bias on a cannabis-modified Stroop task (Field, 2005). Similarly, light social drinkers had less attentional bias to alcohol cues than heavy drinkers (Field, Christiansen, Cole, & Goudie, 2007; Townshend & Duka, 2001). In the current assessment, SDIs were impaired on all aspects of the task, registering significantly slower responses on both trial conditions. This is suggestive of a more globalized inefficiency in these individuals that is not unique to the cocaine-related stimuli, potentially relating to increased age, lower IQ or general cognitive impairment from prolonged stimulant use. However, the higher interference scores in these participants, demonstrating significantly slower responses on cocaine versus neutral trials, are indicative of greater content-specific impairment as well. 78 Chapter 3. Absence of cocaine cue bias in recreational stimulant users The decrease in activation in the IFG and OFC seen in the recreational users also suggests that these words do not hold the same salience as they do for the dependent users. The IFG is thus not equivalently recruited in the recreational users, as the need to inhibit an initial attentional bias is less prevalent in these individuals. Increased activation in the SDIs compared with recreational users could reflect greater effort being exerted to resist the distracting cocaine words, represented via increased interference scores on the task. Higher prefrontal activity in these participants could also be due to the increased salience of the words for these individuals, resulting in greater neuronal excitation. However, it should be noted that in only one cluster in the IFG did SDIs show greater BOLD activation than control individuals. Thus, the significant contrast was primarily between the dependent and recreational users of cocaine, rather than between dependent individuals and control participants. This result is surprising given the significant attentional bias to these cues in the SDIs compared with the control group, and previous evidence showing heightened BOLD response in response to drug-related cues in dependent individuals (Ersche, Bullmore, et al., 2010). Resistance to drug-related attentional bias as measured by the Stroop has previously been cited as an indicator of successful abstinence efforts in treatment seekers (Brewer et al., 2008; Carpenter et al., 2006; Marissen et al., 2006). In the brain, impairments in response inhibition manifested as abnormal activation in the frontal pole and cingulate cortex, thought to correspond with the increased cognitive demand required to override an initial emotional reaction to the drug words (Ersche, Bullmore, et al., 2010; Goldstein et al., 2009; Goldstein, Tomasi, Rajaram, et al., 2007; Wexler et al., 2001). The inferior frontal gyrus in particular has been found to exhibit increased activation in response to drug cues, and is known to be associated with cognitive control and the inhibition of prepotent responses (Bunge et al., 2001; Ersche, Bullmore, et al., 2010). 79 Chapter 3. Absence of cocaine cue bias in recreational stimulant users Previous work with the current participant groups has shown differences in cortical structure, with recreational users having significantly greater volume in the OFC, while dependent users showed a significant reduction in OFC grey matter, as well as in the posterior insula (Ersche, Jones, Williams, Smith, et al., 2013; Ersche, Jones, et al., 2012). Similar dissociation in OFC activation during the cocaine-word Stroop task between dependent and recreational users of cocaine may reflect the structural differences in this area, which could be serving as a protective factor against the development of dependence in recreational users. In the previous chapter, increased frontal volume was associated with more efficient cognitive processing, and it is possible that the enlarged OFC in the recreational users compared with control and SDIs resulted in them having to recruit less cortical activity during task performance, rendering the task less cognitively demanding. Rather than being at an earlier stage in the development towards dependence, we believe that these individuals are able to maintain a recreational pattern of cocaine use without devolving into full-fledged dependency. The cognitive, behavioural, structural and functional data all point to a unique pattern of use in these individuals that is distinct from those who are dependent on cocaine (Ersche, Jones, Williams, Smith, et al., 2013). Additionally, data from the biological siblings of SDIs show very different results, displaying cognitive, personality and structural traits that are most similar to those of their dependent siblings, despite never having been dependent on drugs (Ersche, Jones, et al., 2012; Ersche, Turton, et al., 2012, 2010). Thus, these findings suggest two possible important qualitative differences between dependent and recreational stimulant users. Underlying and potentially predisposing factors for stimulant dependence, such as decreased cognitive efficiency and impaired impulse control, may be present in the former, as well as in their non-drug abusing siblings, while these potentially predisposing factors are not present in recreational users. Conversely, recreational users may exhibit 80 Chapter 3. Absence of cocaine cue bias in recreational stimulant users resilience against the development of dependence, including neurobiological changes such as increased orbitofrontal cortex size and efficiency that are not evident in dependent users or their siblings. This pattern of qualitative differences would seem to make it unlikely that recreational users are merely at an earlier stage on an ultimate trajectory to dependence. In the current cocaine-word Stroop task, it is difficult to parse out whether the differences between recreational and dependent users are due to impaired executive control in the SDIs in response to cocaine stimuli, or a global increase in cognitive control in the recreational users. Future studies should include a non-drug related evocative word condition to test whether the difference is specific to cocaine-related words or is also present for other salient emotional stimuli. Additionally, the Stroop paradigm has been shown to have poor internal reliability (Cools et al., 2007), and studies employing objective indices of attentional bias, such as eye-tracking, should be administered to test the reproducibility of this effect. The findings reported here focus on the responses of qualitatively different groups of cocaine users – dependent and recreational individuals – to drug-related stimuli. Given the known abnormalities in the mesolimbic dopamine circuitry and corresponding differences in reward system responding associated with heavy stimulant use, comparing reactions to a more general reinforcer could help illuminate how wide-spread these differences in reward responsivity are in dependent and recreational users. Additionally, reintroducing the sibling participants to this analysis would enable a comprehensive comparison of predating and consequential differences in the brain and behaviour involved in stimulant use and dependence. This analysis is pursued in the following chapter. 81 Chapter 4. Decreased reward sensitivity protects against stimulant dependence Chapter 4. A Comparison of Endophenotype and Stimulant Exposure Effects in Reward Sensitivity 1. Introduction In addition to the ‘top-down’ cognitive impairments seen in dependent stimulant users, there are also known dysfunctions in ‘bottom-up’ processes, such as atypical reward responding associated with altered dopamine activity in the striatum (Schott et al., 2008). A growing body of research has suggested that abnormalities in the mesolimbic dopamine reward circuitry may both underlie and be perpetuated by the effects of stimulant drugs acting directly on this system (Blum et al., 2000; Volkow, Wang, Telang, et al., 2008). In the previous section, neural responses to cocaine cues were measured using functional neuroimaging to assess cognitive bias to drug-related stimuli. In the current study, we examined blood oxygen level dependent (BOLD) activation during functional magnetic resonance imaging (fMRI) in response to a more universal (though secondary) reinforcer – money – to determine if changes in general reward responsivity are linked to increased risk for drug dependence, or if abnormalities in reward circuitry are observed only in response to drug cues. The Monetary Incentive Delay task (MID) is a well-validated paradigm that assesses sensitivity to differing magnitudes of financial rewards (Knutson et al., 2000). Larger rewards typically elicit greater activation in the ventral and dorsal striatum during anticipation to respond, as well as the medial orbitofrontal cortex (OFC) (Knutson, Adams, Fong, & Hommer, 2001; Knutson, Fong, Adams, Varner, & Hommer, 2001; Schott et al., 2008). The striatum has long been implicated in reward responding and, as discussed 82 Chapter 4. Decreased reward sensitivity protects against stimulant dependence previously, is also a key target for addiction research, with stimulant drugs acting directly on the dopamine-rich circuitry (Berridge & Robinson, 1998; Everitt & Robbins, 2005; Wise & Bozarth, 1985; Wise & Rompre, 1989). Manipulation of the striatal dopamine system through the use of illicit substances is thought to ‘hijack’ normal reward processing in stimulant-dependent individuals (SDIs), with stimulant drugs becoming over-valued while other rewards, such as food or sex, lose their reinforcing properties (Bjork et al., 2008; Garavan et al., 2000; Goldstein et al., 2007; Jia et al., 2011). Likewise, decreases in grey matter volume and BOLD activation in the OFC, an area involved in reward valuation and decision-making (Bechara et al., 2000; Levy & Glimcher, 2012) have also been observed in addicted individuals, and are potentially a consequence of prolonged stimulant use (Bolla et al., 2004; Ersche et al., 2011; Franklin et al., 2002; Matochik et al., 2003). Previous studies administering the MID to substance-dependent individuals have reported somewhat conflicting results. Studies in stimulant- and cannabis-dependent individuals have shown a hyper-activation of the BOLD response, particularly in the ventral striatum, medial frontal lobes, insula and anterior cingulate cortex (Bjork et al., 2008; Jia et al., 2011; Nestor, Hester, & Garavan, 2010). Conversely, alcohol-dependent individuals exhibit a hypoactivation to reward relative to healthy control volunteers (Beck et al., 2009; Wrase et al., 2007). As with most impairments associated with stimulant dependence, it is unclear whether the differences in activation are an underlying issue, predating and potentially predisposing an individual to find stimulant drugs more rewarding and thus making them more susceptible to the development of dependence, or if the disruption is a result of the effects of stimulant drugs on the brain. In the current study, we compared behavioural performance and neural 83 Chapter 4. Decreased reward sensitivity protects against stimulant dependence responses between all four of the same participant groups from the previous two chapters – dependent stimulant users, their non-dependent biological siblings, recreational users of cocaine, and healthy control volunteers – on a modified MID task during fMRI scanning. We compared BOLD responses to different magnitudes of monetary rewards in a priori designated regions of the striatum and OFC, and additionally completed an exploratory whole-brain analysis. We also adapted the task to include drug-related stimuli in an attempt to determine if responses to drug-specific rewards mirror those seen to money, a more general reinforcer. If abnormalities in reward processing represent underlying risk factors for dependence, we would expect the siblings and SDIs to display similar responses to the money cues. Conversely, if alterations in reward processing are a consequence of the effects of stimulant drugs or an indicator of a willingness to use drugs, we would expect dependent and recreational users to show similar responses, and for both to differ from the control and sibling participants (though due to greater exposure the SDIs might show a greater effect than the recreational users). In the current investigation, we hypothesised that stimulant-dependent individuals would exhibit heightened sensitivity to both monetary and drug stimuli via increases in BOLD activation in the striatum and OFC compared with the other participant groups, as shown in previous studies (Bjork et al., 2008; Jia et al., 2011). We predicted recreational users to also show an increase in activation to reward anticipation compared with control and sibling participants, but not to the same extent as the dependent users due to differences in exposure and drug use severity. Finally, we hypothesised that sibling and control participants would not differ in their responses to either condition. Thus, we predict that sensitivity to reward 84 Chapter 4. Decreased reward sensitivity protects against stimulant dependence will not reflect an endophenotypic trait, but will instead be more suggestive of a potential consequence of stimulant use on the brain’s reward circuitry. 2. Materials and Methods 2.1 Participants Recruitment, screening and demographic information for the four participant groups have been described in the preceding two chapters. Four groups of 50 stimulant-dependent individuals (SDIs), 50 of their non-dependent biological siblings, 27 recreational cocaine users, and 52 unrelated healthy control volunteers took part in the study. In the current investigation, 39 individuals were excluded from the analysis due to excessive head movement, presence of a clinically significant neurological abnormality, or poor performance on the task in which a condition had to be discarded due to no correct responses. This resulted in 140 total participants (control=43, SDI=35, sibling=40, recreational=22). Demographic information for this subset of participants is provided in Appendix C (Table S4.1). 2.2 Behavioural Task Participants completed a modified version of the Monetary Incentive Delay task (Knutson et al., 2000) during fMRI scanning using two types of motivationally salient outcomes: monetary rewards – similar to those used in the original task – and images of drug stimuli. The task was administered in two runs (money, drug), presented in randomised order. Participants were told that they would be paid according to their performance, i.e. the money that they earned during the monetary condition plus an equivalent financial bonus reflecting their performance on the drug condition. 85 Chapter 4. Decreased reward sensitivity protects against stimulant dependence In the task, participants were presented with one of five different cues signalling the trial type. In the money condition, the cue was associated with a large (50 pence) or small (10 pence) reward, or a neutral (no reward) outcome (see Figure 4.1). As in the original MID task, the neutral cue was an empty circle, the small money cue was a circle transected by a single horizontal line, and the large money cue was a circle transected by two horizontal lines. In the drug condition, the rewarding cue consisted of an image of the stimulant-using individual’s drug of choice (either powder cocaine, crack cocaine or amphetamine), while a picture of a water bottle constituted the neutral cue. The rewarding feedback slides depicted an image of an individual self-administering the drug of choice or drinking water for the neutral trial. Cues were presented for 250 ms, followed by a jittered blank anticipation screen for 30005000 ms with a mean of 4000 ms. A target stimulus (white square) was then presented for between 100-400 ms, during which time the participant had to respond in order to receive reward. If the participant responded before the target disappeared, a feedback screen depicting the amount of money won, or an image of a person using their drug of choice, was presented for 1650 ms. If the respondent was too slow, an image of the neutral feedback slide was presented representing a non-win trial – i.e. either the blank circle depicting 0 pence or the image of a person drinking from a water bottle. At the end of the experiment, participants were asked to rate their willingness to pick up either a 10 pence or 50 pence coin off the floor on a visual analogue scale (Always – Never) as a proxy for subjective value of each monetary reward. 86 Chapter 4. Decreased reward sensitivity protects against stimulant dependence Cue 250 ms Anticipation 3000-5000 ms Target 100-400 ms Feedback 1650 ms Figure 4.1. Image displays for the modified Monetary Incentive Delay task for the money and drug conditions. Participants were primed with a cue designating the type of 87 Chapter 4. Decreased reward sensitivity protects against stimulant dependence trial that was about to take place, followed by a jittered anticipation slide and then the target to which they must respond. A feedback slide signifying either a successful or unsuccessful trial was then displayed. Unsuccessful trials were represented by the neutral image conditions. 2.3 Behavioural Analysis Mean response times for each condition were compared between groups using general linear model (GLM) multivariate analyses, with Bonferroni post-hoc corrections for multiple comparisons, in the Statistical Package for Social Sciences (SPSS v.19). A repeated measures GLM was conducted for the drug and money conditions separately, with group (four levels: SDIs, siblings, recreational users and healthy volunteers) as the between-subjects factor and cue type (two levels: reward, collapsed across high and low value stimuli for the money condition, and neutral) as the within-subjects factor. For post-hoc analysis, simple main effects were calculated using relevant variations of this GLM. Subjective monetary valuations of 10 pence and 50 pence, as well as the total amount of money earned, were compared between groups using GLM multivariate analyses with Bonferroni post-hoc corrections. 2.4 Imaging Analysis See image acquisition parameters described in Chapter 2, Section 2.4. FMRI data were analyzed using Statistical Parametric Mapping software (SPM v.8) (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/; London, UK). The first five images were discarded to account for T1 equilibration. Images underwent motion and slice-timing correction and were registered to Montreal Neurological Institute (MNI) standard stereotactic 88 Chapter 4. Decreased reward sensitivity protects against stimulant dependence space. Images were spatially smoothed using a 5 mm Gaussian kernel full-width at half maximum. Scans with movement greater than 1.5 mm in any direction, or enough to cause inter-slice variance, were excluded from analysis. Other scan exclusion criteria included large dropout in the key regions of the basal ganglia and OFC. At the first-level, design matrices modelling BOLD hemodynamic response functions at the time of anticipation (cue onset) and receipt of feedback (outcome) were created for each stimulus type (money large, money small, money neutral, drug, drug neutral). Large and small money trials were combined in contrasts to compare responses across the reward conditions with the money neutral trials. Comparisons between anticipation of reward (money/drug) and neutral trials were conducted, as were comparisons for receipt of reward for successful (win) compared with unsuccessful (no win) outcomes. In total, four contrasts were computed: anticipation of money minus anticipation of neutral; anticipation of drug minus anticipation of neutral; feedback during successful money trials minus feedback during unsuccessful money trials; and feedback during successful drug trials minus feedback during unsuccessful drug trials. In second level analyses, the main effect of each contrast (collapsing across group) was computed using one-sample t-tests. The groups were then compared on each of the four contrasts using analysis of variance (ANOVA) F-statistics. Further contrasts between the participant groups were also run, enabling us to better answer our a priori question of which traits in stimulant dependence are due to potential endophenotype effects and which may be attributed to stimulant use. This included a contrast of those with a personal or family history of drug dependence (SDIs and siblings) to those without (control individuals and recreational 89 Chapter 4. Decreased reward sensitivity protects against stimulant dependence users), as well as an investigation into the effects of stimulant exposure (SDIs and recreational users versus control and sibling participants). Analyses for all contrasts were repeated applying a priori specified regions of interest (ROIs: ventral and dorsal striatum; orbitofrontal cortex), defined using PickAtlas (http://www.nitrc.org/projects/wfu_pickatlas/) with Anatomic Automatic Labelling (AAL) coordinates. We corrected for multiple comparisons, controlling the family-wise error rate (FWE) at the voxel-level. Principal eigenvariates for significant clusters identified during the second-level analyses comparing groups were extracted from SPM and compared post-hoc in SPSS using ANOVA. This was used to determine the direction of differences in BOLD response between groups, but not to make formal statistical interference. These eigenvariates were also used for comparisons with other behavioural variables to determine relationships between functional and behavioural performance, using Pearson correlation coefficients. 3. Results 3.1. Demographic As in the previous analyses with these groups, the participants significantly differed on several measures, including age, gender, education levels and smoking status (Appendix C, Table S4.1). As such, these variables were included as covariates in group comparisons of behavioural and functional imaging variables in SPSS in an attempt to control for these differences. 90 Chapter 4. Decreased reward sensitivity protects against stimulant dependence 3.2 Behavioural There was no significant main effect of task condition on reaction times (F(3,136)=1.098, p=0.350; Table 4.1). However, we detected a significant group-by-condition interaction (F(3,9)=2.400, p=0.012). Analyses of simple main effects revealed that within the dependent and recreational groups, responses to the money trials were significantly faster than to neutral trials (dependent: F(1,34)=13.119, p=0.001; recreational: F(1,21)=5.799, p=0.025). This effect was also present in the control group at trend level (control: F(1,42)=3.350, p=0.074), but not in the sibling group (F(1,39)=1.296, p=0.262). There were no significant differences in response latencies among any of the groups on the drug versus drug neutral trials, and there was no main effect of group on global response times. There was a significant difference in the total money earned between the four groups (F(3,136)=2.741, p=0.011), with SDIs obtaining significantly more money than recreational users at Bonferroni corrected p<0.05. The four groups also trended towards a difference in their self-reported likelihood of picking up a 10 pence coin off the ground (F(3,136)=1.993, p=0.061), used as a subjective measurement of the value attributed to the two rewards presented in the task (Figure S4.1). This effect was driven by the sibling participants rating themselves as less likely to pick up 10 pence off the floor than the other three groups; however, this did not survive Bonferroni post-hoc comparisons. There was no difference between groups in subjective valuation for the 50 pence reward (F(3,136)=0.891, p=0.516). 91 Chapter 4. Decreased reward sensitivity protects against stimulant dependence Table 4.1. Behavioural performance differences on the Monetary Incentive Delay task between groups Behavioural Results Money cue mean RT (ms) Money neutral cue mean RT (ms) Drug cue mean RT (ms) Drug neutral cue mean RT (ms) Money won (£)* 10 pence value (% pick up) 50 pence value Control n=43 Mean (SD) Sibling n=40 Mean (SD) Recreational n=22 Mean (SD) Dependent n=35 Mean (SD) F P 205.52 (17.05) 209.17 (21.75) 208.28 (18.14) 205.54 (17.21) 9.30 (1.08) 65.05 (29.64) 76.70 (23.80) 209.49 (22.40) 211.59 (24.51) 215.74 (27.18) 217.48 (27.11) 9.32 (0.91) 49.35 (35.38) 67.42 (31.98) 201.38 (20.32) 206.64 (23.48) 203.30 (21.53) 203.54 (23.23) 9.08 (1.16) 55.36 (32.20) 75.59 (22.18) 206.92 (26.10) 217.83 (30.91) 213.47 (32.06) 217.43 (31.20) 9.89 (1.03) 70.54 (28.88) 79.89 (25.77) 0.365 1.065 1.322 1.539 2.741 1.993 0.891 0.921 0.389 0.245 0.160 0.011 0.061 0.516 *Significant group difference at p<0.05. In Bonferroni post-hoc tests, dependent individuals won significantly more money than recreational users, and sibling participants trended towards ranking a 10 pence coin as less desirable. Analysis corrected for age, gender, education levels and smoking status. 92 Chapter 4. Decreased reward sensitivity protects against stimulant dependence 3.3 Neuroimaging Anticipation of Monetary Reward Analysis of the monetary anticipation contrast (money minus no money trials) revealed significant activation in the right medial OFC and putamen using ROI analyses among all participants (both p<0.05; Figure 4.2; Table 4.2). However, testing the group-by-condition interaction, no significant differences emerged between the four groups in activation during anticipation of the monetary reward in the OFC or striatum. There were also no significant group effects at the whole brain level that survived FWE correction. Additionally, no significant differences arose that survived FWE correction when concentrating on the a priori contrasts testing potential endophenotype effects (those with a personal or family history of dependence versus those without) or the effects of stimulant drug use (stimulant users versus non-users). In the sibling participants, reward-related speeding (i.e. the difference in reaction time between the neutral and money conditions) significantly negatively correlated with activation in the striatum (right putamen: r=0.381, p=0.015; left putamen: r=0.348, p=0.028), such that heightened anticipatory putamen activity corresponded to relatively slower responses on the money compared with the neutral trials. This effect did not emerge in any other participant group, and no other behavioural measures correlated with BOLD activity. 93 Chapter 4. Decreased reward sensitivity protects against stimulant dependence Figure 4.2. Striatal activation amongst all participants during anticipation of money versus neutral trials using region of interest analysis (MNI coordinates (x,y,z) mm: -8, 12, 7). There were no significant differences in activation in either cluster between participant groups. Receipt of Monetary Reward Analysis of the money feedback contrast (successful minus unsuccessful trials) revealed significant activation in the left caudate, bilateral putamen and medial OFC across groups using ROI analyses (all p<0.05; Table 4.2). Across all participants, activation in the caudate was significantly correlated with the amount of money won on the task (r=0.171, p=0.043); no other behavioural measures correlated with BOLD activity. No regions survived FWE correction for the group-by-condition interaction. Likewise, there was no significant activation supporting an endophenotype effect during feedback on money trials. However, there was a significant difference in BOLD response in the OFC ROI (FWE p<0.05) between those with and without a personal history of stimulant exposure: the control and sibling participants exhibited a significant relative increase in activation bilaterally in the medial OFC compared with SDIs and recreational users (Figure S4.2). However, during post-hoc analysis no differences survived Bonferroni post-hoc correction. 94 Chapter 4. Decreased reward sensitivity protects against stimulant dependence Table 4.2. Significant cluster activation among participants during money conditions: anticipation of money compared to anticipation of neutral trials, and feedback for successful versus unsuccessful money trials. Anticipation Money – Neutral All participants ROI striatum FWE p<0.05 Right putamen Left putamen All participants ROI OFC FWE p<0.05 Right medial orbitofrontal cortex Feedback Money Successful – Unsuccessful All participants ROI striatum FWE p<0.05 Right putamen Left putamen Left caudate All participants ROI OFC FWE p<0.05 Left medial orbitofrontal cortex Cluster Size Peak Voxel T Post-hoc ANOVA F Post-hoc ANOVA P 552 521 30, 17, 4 -27, 14, 4 10.36 9.51 1.70 1.68 0.113 0.120 170 12, 35, -11 6.56 0.58 0.769 Cluster Size Peak Voxel T Post-hoc ANOVA F Post-hoc ANOVA P 381 301 69 27, -10, 10 -30, -10, 1 -18, -16, 22 7.48 8.55 6.63 1.09 1.20 0.63 0.374 0.305 0.728 183 -3, 41, -14 6.86 0.31 0.950 Anticipation of Drug Reward Analysis of the drug anticipation contrasts revealed a significant cluster in the right medial OFC (FWE p<0.05), but no activation in the striatum with ROI analyses among all participants. However, in the group-by-condition interaction, there was significantly different activation in the right putamen (F(3,136)=4.042, p<0.001) but no interaction in the OFC. This effect was largely driven by the SDIs, who exhibited an increase in activation in this region compared with their siblings and recreational users, while the sibling participants demonstrated a decrease in activity compared to SDIs and controls (Figure 4.3). There was also a significant group-by-condition interaction in the left middle cingulum, stretching into 95 Chapter 4. Decreased reward sensitivity protects against stimulant dependence the left supplementary motor area, and vermis at the whole-brain level (FWE p<0.05), with effects evident in the same direction (drug users over-activating and siblings under-activating compared with all of the other groups). There was no correlation between behavioural performance and BOLD activation in any cluster during the anticipation condition. Examining the contrasts focused on our hypotheses, the combined stimulant-using group (recreational and dependent) showed a significant increase in activation in the right OFC ROI compared with the combined sibling and control group (Figure S3). This effect was strongest compared with the siblings (recreational: p=0.08; SDI: p=0.020, Bonferroni corrected); the controls did not differ from either of the drug-using groups in post-hoc analyses. There was no effect of stimulant exposure in the striatum. At the whole-brain level, an effect in the same direction was also evident in the left angular gyrus, with the stimulant-using participants demonstrating greater activation than the combined sibling and control group (F(3,136)=3.17; FWE p=0.004). In post-hoc analyses, the recreational users had significantly greater BOLD activity than the siblings (p=0.006, Bonferroni corrected); no other post-hoc comparisons reached significance. There were no differences relating to an endophenotype effect. 96 Chapter 4. Decreased reward sensitivity protects against stimulant dependence A) B) 97 Chapter 4. Decreased Reward Sensitivity Protects Against Stimulant Dependence Figure 4.3. A) Activity in the left middle cingulum (MNI coordinates (x,y,z) mm: -6, 11, 40), which significantly differed between participant groups during anticipation of drug–neutral trials at the whole-brain level. This was driven by dependent individuals having greater activity in this area than all of the other three groups, as well as an increase in control participants compared to the sibling individuals. Other regions that showed a significant group effect during the drug anticipation contrast were the left precentral gyrus/supplementary motor area and in the vermis. B) After a region of interest analysis focusing on the striatum, an additional cluster emerged in the right putamen (MNI coordinates: 30, 17, 4) that significantly differed between participants during the contrast. Again, dependent individuals showed greater activity compared with their siblings and recreational users. Controls also displayed greater activity than the sibling participants. Receipt of Drug Reward Analysis of the drug feedback contrast (successful minus unsuccessful trials) revealed significant activation in the left and right putamen and left OFC using ROI analyses across all participants (all p<0.05; Table 4.3). There were no significant group-bycondition interactions and no significant effects in the planned comparisons relating to either an exposure effect or an endophenotype effect. Activation during drug feedback did not correlate with any behavioural measures. 98 Chapter 4. Decreased Reward Sensitivity Protects Against Stimulant Dependence Table 4.3. Significant cluster activation among participants during drug conditions: anticipation of drug compared to anticipation of neutral trials; and feedback for successful versus unsuccessful drug trials. Anticipation Drug – Neutral Cluster Size Peak Voxel T Post-hoc ANOVA F Post-hoc ANOVA P -6, 11, 40 12.38 5.56 <0.001 -30, 5, 40 4.90 5.11 <0.001 6, -58, -11 4.69 6.05 <0.001 30, 17, 4 9.33 4.04 <0.001 12, 50, -14 3.33 1.52 0.164 Cluster Size Peak Voxel T Post-hoc ANOVA F Post-hoc ANOVA P 407 381 -21, 8, -5 21, 11, -5 9.20 8.90 0.58 0.61 0.768 0.746 120 -3, 44, -14 6.22 0.46 0.862 ANOVA FWE p<0.05 Left middle cingulum* 65 Dependent > Control: p=0.025 Dependent > Sibling: p<0.001 Dependent > Recreational: p=0.010 Control > Sibling: p=0.011 Left supplementary motor area* 2 Dependent > Sibling: p<0.001 Control > Sibling: p=0.013 Recreational > Sibling: p=0.001 Vermis* 14 Dependent > Sibling: p<0.001 Dependent > Recreational: p=0.012 Control > Sibling: p=0.008 All participants ROI striatum FWE p<0.05 11 Right putamen Dependent > Sibling: p<0.001 Dependent > Recreational: p=0.075 Control > Sibling: p=0.010 All participants ROI OFC FWE p<0.05 Right medial orbitofrontal cortex* 1 No significant group differences Feedback Drug Successful – Unsuccessful All participants ROI striatum FWE p<0.05 Left putamen Right putamen All participants ROI OFC FWE p<0.05 Left medial orbitofrontal cortex *Significant group difference at p<0.05. 99 Chapter 4. Decreased Reward Sensitivity Protects Against Stimulant Dependence 4. Discussion A test of reward-responsivity was administered to stimulant-dependent individuals, their non-dependent biological siblings, recreational users of cocaine, and unrelated healthy control volunteers in an attempt to determine whether abnormalities in reward system functioning that commonly accompany stimulant dependence are due to underlying impairments or are the result of long-term stimulant use. Stimulant-dependent individuals and recreational users of cocaine both showed a relative increase in activation during anticipation of stimulant cues compared with non-drug-using control and sibling participants, particularly in the OFC. SDIs also demonstrated an additional increase in striatal activation in response to these stimulant cues. However, both drug-using groups exhibited a relative under-activation compared with that of non-drug-using participants in response to the receipt of a monetary reward. These findings suggest a potential effect of stimulant drugs like cocaine on the mesolimbic dopamine circuitry, and might support the theory of a ‘hijacking’ of the reward system by drugs of abuse, rendering other non-drug reinforcers less effective. 4.1 Effects of Stimulant Use Differences in activity in reward circuitry in the stimulant-using participants – with an increase in activation in response to drug cues, but a decrease in activity upon receipt of a monetary reward – may be a consequence of their drug use, resulting in a disruption of the mesolimbic striatal dopamine circuitry. Alternatively, these varying sensitivities to reward may be a contributor to the initiation of stimulant use, present only in those who are more likely to try drugs. It is notable that the primary group differences between drug-using and non-using participants in response to both types of reward stimuli were in the OFC. This was a 100 Chapter 4. Decreased Reward Sensitivity Protects Against Stimulant Dependence primary region of interest due to its involvement in value (Levy & Glimcher, 2012) and being an area known to be particularly affected by stimulant use (Ersche, Williams, Robbins, & Bullmore, 2013). However, it is somewhat perplexing that the dependent and recreational users demonstrated similar activation in this region on the current task given the significant differences in activation in the area these same individuals displayed in the previous chapter in response to the cocaine Stroop task. Additionally, these same participant groups have also shown significant differences in grey matter volume in this region, with SDIs having a decrease in cortical volume while the recreational users showed an increase in OFC grey matter (Ersche, Jones, Williams, Smith, et al., 2013). While both drug-using groups demonstrated increased OFC activation during anticipation of the drug trials compared with the non-drug users, further differences in activity occurred in the dependent individuals; SDIs showed greater activation in the striatum, left middle cingulum and vermis compared with recreational users. This may be suggestive of a potential dose effect in the SDIs given their greater exposure to cocaine, or this may be indicative of a ‘dependence effect’, reflecting the different patterns of stimulant use in the two drug using groups. This finding is supported by the recreational users’ notable lack of a significant response to cocaine cues in the emotional Stroop task reported in Chapter 3. Curiously, there was no difference in activation between the groups during feedback for drug rewards. It is possible that after prolonged stimulant use, it is only the anticipation of reward that is arousing for stimulant users, while the notice of receipt no longer elicits a significant response. Supporting this, it was only during the feedback condition for money that there was a difference between the stimulant using and non-using groups, with the sibling and control individuals exhibiting a higher BOLD signal. This finding supports the incentive salience model of reward processing, where the cues predicting a 101 Chapter 4. Decreased Reward Sensitivity Protects Against Stimulant Dependence reward come to be over-valued, taking on the reinforcing properties of the stimulus itself (Robinson & Berridge, 1993). Thus, it is potentially only in anticipation of a reward, prompted by the associated cue, that objects are valued, particularly for drug-associated stimuli. However, previous investigations of the MID in stimulant users have reported conflicting results, with SDIs exhibiting a relative increase in activation to monetary reward receipt compared with control individuals (Bjork et al., 2008; Jia et al., 2011). 4.2 Evidence for an Endophenotype Notably, there were no behavioral or functional responses that were uniquely present in both the sibling and stimulant-dependent groups that might have been suggestive of an endophenotype trait. This is in striking contrast to the results reported in Chapter 2, as well as previous investigations in these participant groups in which they displayed evidence of similarities suggestive of an endophenotype, including impulsivity and poor motor control (Ersche, Jones, et al., 2012). However, the different nature of the task used in the current investigation – targeting a reward-related motivation to respond – may explain this unique finding. Interestingly, during anticipation for the drug cues the sibling individuals not only displayed a decrease in activation compared with the SDIs, they also showed a relative decrease in activity compared with the control participants. This may be due to an aversion to the drug-related stimuli due to their experiences with their stimulantdependent brothers and sisters (Barnard, 2005). Supporting this specificity, during the money trials striatal activation in the siblings was no different from that of control volunteers. Prior research in individuals with a family history of alcohol dependence has shown a relative increase in striatal dopamine receptor availability in family-history positive participants (Volkow et al., 2006). This increase was proposed to potentially lead 102 Chapter 4. Decreased Reward Sensitivity Protects Against Stimulant Dependence to a reduced sensitivity for drug reinforcement, acting as a potential protective factor against addiction. In the current study, there do not appear to be any general abnormalities in striatal activity in the sibling individuals, however, any potential striatal dopamine effects similar to those listed above may be limited to drug-specific cues. 4.3 All Group Comparisons Among all participant groups, there was significant activation in the striatum and orbitofrontal cortex in response to rewarding stimuli. This supports the general notion that these areas are crucial for reward processing; it also importantly demonstrates that the current version of the MID accesses the same circuitry as previous investigations using variants of the task (Beck et al., 2009b; Bjork et al., 2008; Knutson, Adams, et al., 2001; Schott et al., 2008). However, there were few differences in activation between groups in this area, limited to the drug anticipatory condition only. The lack of differences in striatal activation was surprising and somewhat conflicting with previous studies in stimulantdependent individuals (Bjork et al., 2008; Jia et al., 2011). The wider and more diverse spread of participant groups in the current investigation may have washed out this effect during the four-group comparisons, particularly as the recreational users fell between the control and dependent participants in their activation during the money anticipation condition, not differing from either group. Shortcomings of this investigation include the ranging demographic differences between the participant groups. Although we included these demographic data as covariates in the analysis, better matching for these variables during recruitment could be improved in future studies. Furthermore, it was not possible to directly compare responses to drug and money rewards within the participant groups due to inherent differences in task design 103 Chapter 4. Decreased Reward Sensitivity Protects Against Stimulant Dependence between the two conditions, thus preventing a direct contrast of responsivity to these two types of reinforcers. Prior research has suggested that with chronic use, dependent stimulant users become increasingly more sensitive to the reinforcing properties of their drug of choice, but relatively less sensitive to other more generic rewards, such as money or food (Aigner & Balster, 1978; Opris et al., 2009; Woolverton & Anderson, 2006). Our results tentatively support this hypothesis, however, going forward, it would be illuminating to test this theory more directly by comparing BOLD response to drug and non-drug rewards in these groups. Furthermore, it is possible that money cues may also have attained drugrelated salience for dependent individuals through the continual purchasing and procurement of illicit substances. Thus, it would be beneficial to look at primary unconditioned reinforcers, such as food or erotic images, which do not suffer from this confound. 104 Chapter 5. Reward valuation in addictive disorders Chapter 5. Reward Valuation and Incentive Motivation in Addictive Disorders 1. Introduction In the previous chapter, responses to a universal conditioned reinforcer, money, and drugspecific cues were examined separately. Stimulant-dependent individuals (SDIs) exhibited an increase in activation to both types of stimuli, suggesting a potentially hypersensitive reward system. This heightened responsivity may place them at a greater susceptibility to first seek out rewarding drug-related experiences and later contribute to maintained use despite negative consequences, as is characteristic of dependence. However, these two different reinforcers were examined separately, preventing a direct comparison of responses to each type of reward. Prior research has suggested that with chronic use, dependent drug users become increasingly more sensitive to the reinforcing properties of their drug of choice, but relatively less sensitive to other more generic rewards, such as money or food (Aigner & Balster, 1978; Opris et al., 2009; Woolverton & Anderson, 2006). This distinction has been used in the debate over the two different reward dysfunction theories involved in stimulant-dependence, the reward deficiency argument (Bjork et al., 2008; Blum et al., 2000) and the theory of incentive sensitization (Robinson & Berridge, 1993). As reported in the General Introduction, in the former, individuals at risk for substance dependence are thought to have a generally under-active reward system, underpinned by a decrease in dopamine D2 receptor availability in the fronto-striatal circuitry. This can then lead to ‘self-medication’ with drugs or other types of dopamine-boosting reward substances, such as high-caloric foods or sex. Further 105 Chapter 5. Reward valuation in addictive disorders down-regulation of dopamine receptors in the striatum and prefrontal cortex due to the chronic effects of drug use can exacerbate the problem, resulting in greater amounts and more potent substances being required to activate the mesolimbic reward system. If this were the case, SDIs would be thought to show a general decrease in responding to universal reinforcers, both compared with control individuals and in contrast to drug-related cues. In incentive sensitization, individuals at risk for substance dependence are thought to have a general over-arousal to rewarding stimuli, leaving them particularly susceptible to the reinforcing properties of activities that tap into the mesolimbic dopamine circuitry. These individuals are generally more impulsive and are drawn towards acutely rewarding stimuli, exhibiting traits of ‘waiting’ impulsivity (Dalley et al., 2011), including steeper delayed reward discounting. If this were the case, SDIs would demonstrate heightened responsivity to all reinforcing cues compared to control individuals. Results from the previous chapter provide support for this theory, as the dependent participants exhibited an increase in responding to both types of reward cues – drugs and money – particularly compared with their biological siblings and recreational users of cocaine. However, a potential caveat of that prior study is the possibility that the money cues may also have attained drug-related salience for dependent individuals through the continual purchasing and procurement of illicit substances. Thus, it is also important to look at more primary unconditioned reinforcers that have no associations with drug use. The question then arises as to how responding to various different types of rewards – drug and non-drug – might vary within an individual with stimulant dependence, and how this might differ from the responses of non-dependent individuals. 106 Chapter 5. Reward valuation in addictive disorders The majority of prior research on incentive motivation in SDIs has either examined responses only to drug-related cues (Goldstein, Tomasi, Rajaram, et al., 2007; Volkow, Wang, Fowler, et al., 2008; Volkow et al., 2006), or they have explored SDIs’ reactivity to a single other type of salient image (i.e., money, erotic cues or non-specific affective images) (Aguilar de Arcos et al., 2008; Aguilar de Arcos, Verdejo-Garcia, Peralta-Ramirez, Sanchez-Barrera, & Perez-Garcia, 2005; Asensio et al., 2010; Beck et al., 2009; Bjork et al., 2008; Gerra et al., 2003; Goldstein, Tomasi, Alia-Klein, et al., 2007; Goldstein et al., 2007; Wexler et al., 2001; Wrase et al., 2007). Very few of these studies have directly compared responding to drugrelated cues with that of other types of more organically rewarding stimuli (Garavan et al., 2000; Goldstein et al., 2009). However, one good example of such research is Garavan and colleagues’ study comparing neural responses to an explicit sex film and a film depicting cocaine use (Garavan et al., 2000). Cocaine-dependent individuals exhibited significantly greater activation for the cocaine film, particularly in reward processing areas such as the anterior cingulate cortex and ventral striatum. Conversely, healthy control participants were significantly more aroused by the sex film, both in contrast to the drug film and compared with the cocaine users’ reactions. While the areas of activation in response to the sex film were similar in both subject groups, as well as overlapping with the activated areas in cocaine-dependent individuals viewing the drug film, the control individuals demonstrated more widespread global activity, as well as significantly greater levels of activation in these regions. This suggests that ordinarily salient stimuli are still excitatory for SDIs and activate the same reward system as cocaine cues, but to a lesser degree and not to the same spatial extent as for drug-naïve controls. Therefore, this study suggests that reward processing in SDIs, though similar for both cocaine and other salient stimuli, appears to be blunted in response to non-drug reward cues. 107 Chapter 5. Reward valuation in addictive disorders Still, it is difficult to discern whether stimulant-dependent individuals’ abnormal reward responses are due to a pre-morbid system abnormality or are a direct result of the effects of stimulant use on the mesolimbic dopamine system. In the previous chapters, we aimed to address this question by testing the non-dependent biological siblings of SDIs and recreational users of cocaine in an attempt to identify possible risk and protective factors involved in dependence. In the current section we will explore this question by comparing SDIs’ responses to different types of rewards with those of a group of individuals who potentially qualify as having another kind of addictive disorder, but without the external elevation of striatal dopamine levels caused by stimulant drugs: obese individuals with binge eating behaviours (OBE). Parallels between the diagnostic pathology, as well as underlying neurobiological abnormalities, have been made between SDIs and OBEs, suggesting that the latter may have a type of ‘food addiction’ (Davis & Carter, 2009). Binge eating is defined as: “recurring episodes of eating, in a discrete period of time, an amount of food that is definitely larger than most people would eat during a similar period of time [with a]…lack of control during the episodes” (American Psychiatric Association, 2000). The Diagnostic and Statistical Manual of Mental Disorders’ criteria for binge eating disorder are reminiscent of those for drug dependence, including an escalation and loss of control over consumption of the substance, and a continuation of the behaviour despite personal distress, and health, social, legal or financial problems. Neurocognitive similarities also abound, with shared dysfunction identified in fronto-striatal circuitry involving dopamine and opioid neurotransmitter systems in both groups (Baldo & Kelley, 2007; Volkow, Wang, Fowler, et al., 2008; Wang et al., 2004). Highly palatable foods, such as those high in fat and sugar, also tap into the same 108 Chapter 5. Reward valuation in addictive disorders mesolimbic dopamine system as drugs of abuse, implicating dysfunction in this area in both conditions (Rada et al., 2005; Small et al., 2003). By examining this separate set of individuals who theoretically have an alternative type of addictive disorder, it will be possible to control for the potential neurotoxic effects of longterm stimulant use on the mesolimbic dopamine reward circuitry. Additionally, measuring responses to a variety of different types of incentive stimuli, including general and drugspecific ones, will enable a differentiation between the two theories of reward processing by testing whether SDIs and OBEs show a general over-activation and arousal in response to any kind of rewarding stimuli, or if they demonstrate atypical responding only to their particular substance of choice. In the current study, a novel reward incentive task was administered to assess responses to a variety of different salient stimuli, including cocaine and alcohol drug cues, money, sweet and savoury high caloric foods, erotic images and pleasant nature scenes. If a system-wide down-regulation in the mesolimbic dopamine circuitry were present, we would predict that SDIs and OBEs would exhibit diminished responses for conventionally rewarding stimuli, represented by slower reaction times, but have faster responses to cues of their ‘drug of choice’. Conversely, an up-regulation of the dopamine receptors would hypothetically result in all salient stimuli carrying greater incentive value and eliciting quicker responses. Additionally, by testing OBEs’ responses and comparing their reactions to food cues with SDIs’ responses to cocaine stimuli, we would be able to more confidently establish whether these impairments were due to the dopaminergic effects of the drugs themselves. Finally, similar decreases in arousal to classically motivating images, both compared with healthy 109 Chapter 5. Reward valuation in addictive disorders controls and against each population’s substance of choice, would further support the notion of addiction spectrum disorders. 2. Materials and Methods 2.1 Participants Fifty-four individuals from three different participant groups were recruited: cocainedependent individuals (n=11), obese persons with binge eating behaviours (n=23), and healthy control volunteers (n=20). All participants were between the ages of 20 and 65, able to read and write in English, and provided written informed consent for participation. Stimulant-dependent participants were recruited from a concurrently running study on cocaine-dependent men (NREC10/H0306/69, PI: KD Ersche). At the end of this initial testing session, the men were offered the opportunity to stay for an additional half hour to complete another computerized test (the reward valuation task) and some follow-up questionnaires. Eleven out of the thirty men approached agreed to stay and take part. These men all met DSM-IV-TR criteria for cocaine dependence and all tested positive for stimulant drugs at the onset of the study testing day, confirming use within the last 72 hours; none were actively seeking treatment for cocaine dependence. Participants with binge eating behaviours were recruited from a subject pool at GlaxoSmithKline from a previous investigation on obese individuals with binge eating behaviours. Binge eating was initially assessed using the Binge Eating Scale (BES) (Gormally, Black, Daston, & Rardin, 1982) for inclusion in the original GlaxoSmithKline study and participant pool. This questionnaire, along with other measures of binge and disordered eating behaviours, was re-administered upon enrolment in the current 110 Chapter 5. Reward valuation in addictive disorders investigation to confirm presence of binge eating. Twenty-three individuals from the participant pool took part in the study; however, five were excluded from analyses as their current BES scores did not meet criteria for binge eating disorder. Control participants were recruited from the local community and had no history of drug or alcohol abuse, or disordered eating. One individual was excluded from the analysis for having a BMI over 30, which placed them into the obese range. 2.2 Behavioural Task The reward valuation task is a novel computerized test modelled on the Monetary Incentive Delay task (Chapter 4) (Knutson et al., 2000). Participants were first primed for 3000 ms with one of six different types of salient images (alcohol, cocaine, erotic pictures, high caloric foods, money, pleasant nature scenes) or a neutral control image (furniture). After the prime, a short jittered delay period from 500-1500 ms was presented, followed by a separate distinct target cue to which the participants had to respond as quickly as possible by pressing the ‘Enter’ key (see Figure 5.1). Participants were given feedback for 1000 ms after each trial and were rewarded based on their reaction times (RT). Responses under their mean RT resulted in a reward of 20 pence; responses faster than one standard deviation below their personal mean won them one pound. If their response was above the mean RT they won nothing and were urged to try again. Each image category contained six different pictures and presentation was randomized throughout the task. The entire image set was repeated four times for a total of 168 trials. Participants were trained on the task with neutral cues before beginning testing, from which their mean reaction time was determined to compare responding during task performance. 111 Chapter 5. Reward valuation in addictive disorders Prime 3000 ms Anticipation 500-1500 ms Cue 1000 ms Blank 1000 ms Feedback 1000 ms You Win 1 Pound!!! You Win 20 Pence! Try Again! Figure 5.1. Reward valuation task experiment design. In the task, participants were first primed by a rewarding salient picture (alcohol, cocaine, erotic, food, money, pleasant nature scenes) or a neutral image (furniture). They were instructed to respond only when they saw the target response cue, which was presented after a jittered anticipation phase ranging from 500-1500 ms. After the target cue was presented, they had to press the ‘Enter’ key as fast as possible, and they were told that the faster they responded the more money they could win. 112 Chapter 5. Reward valuation in addictive disorders 2.3 Self-Report Measures After the task, participants were asked to rate one image from each of the different categories in regards to pleasantness, arousal, wanting and liking using a series of visual analogue scales – i.e. ranging from ‘Very pleasant’ to ‘Very unpleasant’. Participants completed a series of questionnaires to assess impulsive and sensation-seeking personality traits, as these variables have been linked to greater risk for addictive tendencies and are affected by the fronto-striatal dopamine system (Barratt Impulsivity Scale (BIS-11); Zuckerman Sensation-Seeking Scale (SSS-V)) (Patton, Stanford, & Barratt, 1995; Zuckerman, Eysenck, & Eysenck, 1978). Participants were also administered several questionnaires asking about eating behaviours and attitudes towards food (Eating Disorder Examination-Questionnaire (EDE-Q); Binge Eating Scale (BES)) (Fairburn & Beglin, 1994; Gormally et al., 1982). The Beck Depression Inventory (BDI-II) (Beck, Steer, Ball, & Ranieri, 1996) was administered to judge current mood; education levels and the National Adult Reading Test (NART) (Nelson, 1982) were used as a measure of IQ. Questions asking about the participants’ eating patterns and preferred foods, drug and alcohol use, gambling behaviour and disposable income were also included. 2.4 Analysis Between-groups analyses for response times and subjective ratings were conducted using general linear model (GLM) multivariate analyses. Age and gender were controlled for in GLM analyses on response latencies, as these variables differed between groups and significantly affected group performances on reaction times. Within-group repeated measures analyses were conducted to compare responses to the different variables within each group. Analysis of variance (ANOVA) was used to compare demographic and questionnaire data between groups. Pearson’s correlation analyses were used to determine relationships between 113 Chapter 5. Reward valuation in addictive disorders task performance and self-report data. Significance levels were set at p<0.05 and Tukey tests were used in post-hoc analyses. 3. Results 3.1 Self-Report Data Participants significantly differed on several demographic variables, including age, sex, education levels and IQ scores (Table 5.1). Groups also differed on self-report assessments, including measures of disordered eating behaviour, impulsivity and sensation-seeking. Specifically, obese individuals with binge eating behaviours had significantly higher scores on disordered eating measures than either of the other two groups. Both stimulant-dependent and obese participants also had higher ratings of depression and impulsivity than controls; however, obese individuals had significantly lower sensation-seeking scores than stimulantdependent and control participants. 114 Chapter 5. Reward valuation in addictive disorders Table 5.1. Demographic information and self-report responses for 19 control participants, 18 obese individuals with binge eating behaviour, and 11 stimulantdependent men Demographics Ageac Sex (% male)bc Educationab NARTab BMIac Disposable Income Smoker (% yes) THC (% yes) Gamble (% yes) Questionnaires EDE-Qac BESac BDI-IIab BIS-11ab SSS-Vac Control Mean (SD) OBE Mean (SD) SDI Mean (SD) 28.47 (8.54) 47.4% 17.56 (2.43) 118.65 (5.70) 22.54 (2.46) 46.06 (10.92) 50% 12.89 (1.88) 111.18 (9.10) 35.47 (3.95) 35.11 (8.77) 100% 11.64 (2.25) 106.91 (10.02) 24.87 (3.06) 15.82 9.18 31.77 7.42 80.16 <0.001 0.010 <0.001 0.002 <0.001 £365.56 £670.59 £1030.00 3.04 0.058 36.8% 36.8% 15.8% 61.1% 27.8% 27.8% 81.8% 81.8% 40.0% 2.91 2.02 2.05 0.233 0.364 0.358 27.06 (20.36) 7.33 (3.72) 6.22 (4.52) 63.78 (10.29) 21.50 (5.86) 107.35 (46.66) 22.00 (6.62) 14.18 (8.78) 69.59 (7.97) 13.82 (5.81) 43.56 (48.70) 10.11 (10.32) 16.33 (13.22) 67.44 (8.78) 24.00 (9.32) 12.00 14.27 5.08 3.15 7.48 <0.001 <0.001 0.002 0.024 <0.001 F/χ2 P a Significant difference between control and obese individuals (OBE) b Significant difference between control and stimulant-dependent individuals (SDI) c Significant difference between OBE and SDI National Adult Reading Test (NART), Body Mass Index (BMI), Eating Disorder Examination-Questionnaire (EDE-Q), Binge Eating Scale (BES), Beck Depression Inventory (BDI-II), Barratt Impulsivity Scale (BIS-11), Zuckerman Sensation-Seeking Scale (SSS-V). 115 Chapter 5. Reward valuation in addictive disorders 3.2 Response Time Behavioural Analysis Response times for each of the different conditions were compared between groups using a general linear model multivariate analysis, controlling for age and gender as these variables significantly related to response time differences on the task (Table 5.2; Figure 5.1). After taking into account these variables, there was a significant difference between groups for alcohol (F(2,43)=3.217, p=0.022), money (F(2,43)=3.340, p=0.018), and pleasant nature scenes (F(2,43)=2.806, p=0.038). Reaction times for food stimuli differed at p<0.10 (F(2,43)=2.324, p=0.072). However, in post-hoc analyses, significant differences only emerged between groups for the food condition, with OBE participants responding significantly slower than both controls (p=0.036) and SDIs (p=0.046). Response times for the different stimuli were also compared within each participant group using GLM repeated measures analyses. For controls, there were no significant differences in response times between the different conditions at p<0.05, however, several trended towards significance at p<0.10. This primarily consisted of slower response times for the neutral images (compared to alcohol p=0.083, food p=0.058 and money p=0.064). There were also slower times for the cocaine compared with the food condition (p=0.080). Stimulantdependent participants were significantly faster on the money condition than for all other reward images (compared to cocaine p=0.003, erotic p=0.012, nature p=0.006 and neutral p=0.007; trending at p<0.10 for alcohol p=0.058 and food p=0.085). There was also a significant effect between food and nature images (p=0.048), food and cocaine images (p=0.038), and food and neutral images at p<0.10, with participants responding significantly faster on food images in both contrasts. For the OBE group there were no differences between response times in any of the conditions, except for a difference at p<0.10 between nature and neutral images. 116 Chapter 5. Reward valuation in addictive disorders Figure 5.1. Mean response times for each condition by group. Across the board, obese individuals with binge eating behaviour (OBE) were slower at responding than controls and stimulant-dependent individuals (SDI). There were no differences between controls and SDIs on their response times for any condition. Within the groups, SDIs were significantly faster on the money condition and to a lesser degree in response to food cues, while the control participants were slower overall to the neutral images. The OBE participants did not differ within their responses to the different stimuli. 117 Chapter 5. Reward valuation in addictive disorders Table 5.2. Reaction times for reward image conditions and differences between groups controlling for age and gender Control Mean (SD) Behavioural Results Alcohol* 237.58 (36.36) Cocaine 244.94 (41.66) Erotic 238.18 (37.93) Food 234.34 (33.60) Money* 237.29 (31.43) Nature* 242.93 (35.28) Neutral 247.65 (37.15) OBE Mean (SD) SDI Mean (SD) F P 263.64 (32.97) 260.38 (33.91) 261.74 (28.39) 268.86 (41.01) 263.41 (44.45) 265.77 (41.05) 256.66 (30.31) 241.81 (30.67) 250.48 (33.38) 247.15 (39.75) 239.32 (32.88) 230.59 (32.33) 250.01 (28.76) 251.21 (40.84) 3.22 1.53 1.70 2.32 3.34 2.81 0.75 0.022 0.211 0.168 0.072 0.018 0.038 0.562 * Response times significantly differed between groups at p<0.05 3.3 Subjective Ratings On the subjective ratings, there were group differences for cocaine (F(2,42)=20.451, p<0.001), erotic (F(2,42)=3.981, p=0.008), sweet food (F(2,42)=3.378, p=0.017), and money images (F(2,42)=3.336, p=0.018). The differences for cocaine and erotic trials were driven by the SDIs judging the images significantly more positively than the other two groups (cocaine p<0.001; erotic p=0.002). However, for the sweet food images the group differences were driven by the SDIs rating the images less highly than the other groups (p=0.039). There were no differences between groups for savoury foods. Finally, ratings for the money condition also significantly differed, this time with the control participants reporting that they liked the images less than the other two groups (p=0.034). 3.4 Correlation Analyses Age significantly correlated with reaction times for all conditions except for food and neutral images. Self-reported discretionary income did not correlate with responses to any condition 118 Chapter 5. Reward valuation in addictive disorders and most notably did not correlate with response times for monetary rewards. BMI and ratings of disordered eating behaviour did significantly positively correlate with reaction times on all task conditions, which was most likely driven by the obese participants having significantly slower responses across the board compared to the other two groups. Sensationseeking scores significantly negatively correlated with response times on alcohol, erotic, food, money and nature images. However, sensation-seeking also significantly negatively correlated with age, with younger individuals having higher sensation-seeking scores as well as faster response times overall, so this may have been driven by an age effect. Within each group individually, age correlated with all response times in control participants. BMI also significantly correlated with reaction times on cocaine, food, money and nature scenes in this group. Notably, age and BMI did not correlate in these participants, suggesting that it is not a combined effect of age influencing BMI and response times. Sensation-seeking did not correlate with responses in these individuals. In the OBE group, age did not correlate with response times, however, BMI did still positively correlate with reactions to cocaine, erotic, nature and neutral images. Disordered eating only significantly correlated with responses to food cues, with greater dysfunction relating to slower responses. Interestingly, neither eating disorder nor binge eating scales correlated with BMI, though the two measures did highly correlate with one another. Binge eating scores also positively correlated with alcohol and erotic reaction times in this group. BIS-11 impulsivity scores correlated with reaction times for the food images, but not with any other condition. Sensation-seeking total scores did not correlate with reaction times. In stimulant-dependent men, the only significant correlation that existed was between responses to erotic images and sensation-seeking total scores. 119 Chapter 5. Reward valuation in addictive disorders 4. Discussion In the current investigation, it was predicted that stimulus cues that were judged as more rewarding would elicit faster response times due to either an increase in incentive salience for those stimuli or because of a more generalised decrease in responsiveness to other rewarding cues. Specifically, we hypothesised that participants dependent on cocaine would respond faster for cocaine cues compared to other types of rewarding images, as well as compared with other participant groups, and that obese individuals with binge-eating behaviours would respond relatively faster for food cues. Instead, there were few differences either between or within the groups, and those that did emerge seemed to adhere more to a general pattern of group differences in response times, potentially influenced by age and gender, rather than to the groups’ particular reward preferences. For example, money and food cues elicited the fastest times within SDIs, rather than cocaine trials, and there were no significant differences between groups during the drug condition. Conversely, OBE participants were slower on the majority of trials compared with the other groups, and particularly in response to food cues. Overall, there were trends for both age and BMI to be positively correlated with reaction times on all conditions, while sensation-seeking scores corresponded to faster response times on most task conditions, though this effect may also have been influenced by differences in age on the measure. A potential alternative explanation for results on the task is that rather than being incentivised by the targeted rewarding stimuli, the two clinical participant groups were actually distracted by these specific images, resulting in slower reactions. This would be similar to the cocaineword Stroop effect seen in Chapter 3, with dependent individuals responding significantly slower in the face of cocaine cues. However, within these groups responses to cocaine and 120 Chapter 5. Reward valuation in addictive disorders food trials, respectively, did not differ from the reaction times for other reinforcers, regardless of direction, though higher eating disorder scores in the OBE group did correspond specifically to slower reaction times on food trials. Interestingly, within the groups there were no correlations between subjective ratings for each of the different stimuli and the reaction times elicited in response to them. This could have one of two implications: 1) there is a ‘disconnection’ or uncoupling between the participants’ subjective experiences and their behavioural responses, or 2) the current task may not be sufficiently sensitive to allow a behavioural correlate of individuals’ subjective experiences. The Monetary Incentive Delay task (MID), on which this paradigm is based, rarely produces behavioural performance effects between groups as there is both a hypothetical ceiling and floor effect for response times – i.e. an individual can only respond so quickly on a measure of reaction times, and if a participant is too slow on the task they will not win any money, so they must respond on most trials within a certain amount of time. As such, the MID was designed to be administered using functional magnetic resonance imaging (fMRI), as reported in the previous chapter. Whilst there are typically no behavioural effects present in the task, activation in various reward regions, most notably the ventral striatum and orbitofrontal cortex, often show differences in responding between task conditions, as well as between participant groups. Therefore, it would be interesting to develop the current reward valuation task into an imaging paradigm to determine if there were differences in functional responses to the various stimuli, both within and between the participant groups. The poor matching between the different participant groups in key demographic variables, particularly age and gender, which can significantly affect response times (Tun & Lachman, 121 Chapter 5. Reward valuation in addictive disorders 2008) places a major caveat on the conclusions of this study. Thus, the most stable group effect seen on the task of OBEs responding significantly slower than the other groups may have been driven by the significant increase in age and greater number of female members in this group. However, it should be noted that after controlling statistically for both of these variables there were still significant differences between the groups, with the OBEs again responding more slowly. Additionally, within the control group, BMI significantly correlated with response times on the task. It is possible that this trend could be indicative of an overall flattening of reward responsivity corresponding to increased BMI, similar to the suggested reward deficiency theory. Previous studies investigating responding to a food reward in obese individuals demonstrated an increase in neural activation in fronto-striatal circuitry during anticipation of a food reward, but a subsequent decrease in striatal activation upon receipt (Stice, Spoor, Bohon, & Small, 2008; Stice, Yokum, Blum, & Bohon, 2010). It is possible that the slower response times seen in the OBE group reflect a decrease in motivation for general rewards; however, the absence of a difference in anticipatory excitation to the food rewards somewhat refutes this theory. Again, it would be illuminating to administer the reward valuation task during fMRI scanning to determine if there were any differences in neural activation to the different reward stimuli. On the other hand, the stimulant-dependent individuals were quicker to respond to the money cues and subjectively rated some of the images more highly than the other two groups. This finding is similar to that of the previous chapter, with stimulant-dependent individuals showing a heightened arousal to both types of rewarding cues, potentially reflective of the incentive sensitization theory. Thus, in the obese individuals there appeared to be a general flattening of responses, suggestive of the reward deficiency theory, while the drug users demonstrated behaviours perhaps more characteristic of incentive sensitization. It could be 122 Chapter 5. Reward valuation in addictive disorders that the effect of protracted stimulant use on the dopamine reward system resulted in this sensitization in the cocaine-dependent men, or these differences could still reflect underlying disparities between the two groups. These differences in response patterns between the SDI and OBE groups, the two potential ‘addiction spectrum’ cohorts, tends to contradict the notion of shared abnormalities or underlying risk traits in reward responding in these individuals. However, there are still other potential similarities between the two groups that provide a case for classifying them as similar disorders along a spectrum. This includes shared structural and functional abnormalities in self-control and reward processing regions, such as the lateral and medial frontal cortices (Franklin et al., 2002; Pannacciulli et al., 2006; Volkow, Wang, Fowler, et al., 2008; Volkow et al., 2001; Volkow, Wang, Telang, et al., 2008). In the following chapter we explore this concept through an investigation of structural similarities between the groups. 123 Chapter 6. OFC loss with BMI and cocaine use Chapter 6. Overlapping Decline in Orbitofrontal Grey Matter Volume Related to Cocaine Use and Body Mass Index 1. Introduction Although there was no evidence of behavioural similarities between stimulant-dependent and obese individuals with binge eating behaviours in the previous chapter, there are still shared structural brain abnormalities that may both underlie and be exacerbated by these two conditions. Specifically, grey matter reductions in the OFC have been seen in obese (Gunstad et al., 2008; Pannacciulli et al., 2006; Taki et al., 2008; Walther, Birdsill, Glisky, & Ryan, 2010) and cocaine-dependent individuals (Ersche et al., 2011; Franklin et al., 2002; Liu et al., 1998; Matochik et al., 2003), and deficiencies in OFC glucose metabolism have been reported in both groups (Volkow et al., 1991, 1993, 2001, 2009; Volkow, Wang, Telang, et al., 2008). Additionally, corresponding impairments in associated cognitive processes, such as executive functioning and affective decision-making, are also present in both conditions (Davis, Levitan, Muglia, Bewell, & Kennedy, 2004; Ersche, Clark, et al., 2006; Gunstad et al., 2007; Verdejo-Garcia et al., 2006). As discussed in the General Introduction, the orbitofrontal cortex (OFC) is critically involved in integrating emotion and behaviour, as in cases of reward valuation (Bechara et al., 2000; Levy & Glimcher, 2012). These abilities comprise goal-directed behaviour, actions carried out to achieve a certain objective (Balleine & O’Doherty, 2010). Dysfunction in the OFC can impair these abilities, resulting in behaviour that becomes compulsive, maintained despite attempts to stop or a shift in desired outcome. The OFC is also crucially implicated in the gustatory cortex, providing evaluative judgments on taste (Rolls, 2000). This overlap 124 Chapter 6. OFC loss with BMI and cocaine use between reward valuation and motivated behaviour makes the OFC a key target for impulsive/compulsive disorders, like drug addiction and, more recently, compulsive overeating leading to obesity, as in cases of binge eating. Both conditions can be thought of as behaviours that began as hedonically motivated actions but devolved into undesirable habits, and as such, both have been linked to variations in this region. These neurobiological commonalities, particularly in this key area of the OFC, have led to the discussion of classifying over-eating leading to obesity, as in instances of binge eating, as an addictive disorder (Davis & Carter, 2009) – though this is still considered to be a contentious proposal (Ziauddeen & Fletcher, 2013). However, despite the reported overlap between these behavioural and structural deficits, no direct comparisons of cortical volume between the two conditions have been made. In the current investigation, we used voxelbased morphometry to contrast structure volume in the OFC relating to both body mass index (BMI) and years of cocaine use in a previously collected dataset of stimulant-dependent and matched control participants with BMIs ranging up to obese. We hypothesised that there would be overlapping regions of OFC volume decline corresponding to both years of cocaine use and BMI, both within the stimulant-dependent group, as well as between stimulantdependent and control individuals. 2. Materials and Methods 2.1 Participants We compared structural MRI scans of a previously published sample of 60 healthy control volunteers with BMIs ranging from normal weight to obese (17.8–33.5, mean=25.5), and 60 cocaine-dependent individuals with a similar range of BMIs (16.6–32.7, mean=24.2) and a span of 31 years of stimulant use (mean=9.8 years) (Ersche, Jones, Williams, Robbins, & 125 Chapter 6. OFC loss with BMI and cocaine use Bullmore, 2013; Ersche et al., 2011). Cocaine-dependent individuals were recruited from local drug treatment services and satisfied criteria for stimulant dependence according to the DSM-IV-TR (American Psychiatric Association, 2000). Control participants were free from any neuropsychiatric clinical history and were recruited from the community. Control and cocaine-dependent participants did not differ in terms of age, gender or mean BMI, though there was a significant difference in years of education (Table 6.1). Body-weight from two controls was missing, resulting in 118 total participants. Table 6.1. Demographic information and group differences for 60 cocaine-dependent individuals and 58 healthy control volunteers Demographics Gender (n male: %) Age (years) NART (score) Education (years)* Body mass index Control Mean (SD) Cocaine Dependent Mean (SD) T/χ2 P 46 (76.7%) 32.3 (8.3) 110.0 (7.0) 12.3 (1.6) 25.53 (3.3) 53 (88.3%) 32.5 (8.5) 109.5 (6.9) 11.5 (1.7) 24.22 (4.3) 2.83 -0.12 0.36 2.78 1.83 0.148 0.905 0.716 0.006 0.070 To address the current hypothesis, grey matter data were newly processed by P.S. Jones using voxel-based morphometry (VBM) analysis to take into account the effects of BMI and stimulant use on brain volume. Groups were analysed separately to account for previously discovered differences in brain structure (Ersche et al., 2011), and analyses were focused on the a priori designated region of the OFC. BMI scores were regressed onto OFC volume in all participants, and years of cocaine use were regressed onto OFC grey matter in the cocaine users. We compared affected areas, identifying shared regions of decline associated with both years of cocaine use and BMI, as well as comparing OFC volume between the two groups. 126 Chapter 6. OFC loss with BMI and cocaine use 2.2 Imaging Procedures T1 structural MRI data were obtained using a 3T Siemens Magnetom Tim Trio scanner (Siemens Medical Solutions, Erlangen, Germany) at the Wolfson Brain Imaging Centre, University of Cambridge, UK. One hundred seventy-six scans were collected using a magnetically prepared rapid acquisition gradient echo sequence (MPRAGE) with the following parameters: thickness=1 mm, repetition time=2.30 ms, echo time=2.98 ms, inversion time=900 ms, flip angle=9˚, field of view=240 x 256. Grey matter volume maps from each participant were processed using FSL VBM 1.1 (http://www.fmrib.ox.ac.uk/fsl/fslvbm/index.html). Images were tissue-segmented using FAST (Zhang, Brady, & Smith, 2001), and resulting grey matter volumes were aligned to standard MNI stereotactic space using FLIRT affine registration, followed by non-linear registration using FNIRT (Andersson, Jenkinson, & Smith, 2007a, 2007b; Jenkinson, Bannister, Brady, & Smith, 2002; Jenkinson & Smith, 2001). Images were averaged to create a grey matter template, and each image was then non-linearly re-registered to the group map. Images were smoothed using a full-width half-maximum (FWHM) 2.3 mm Gaussian kernel. Images were analysed in CamBA 2.3.0 (http://www-bmu.psychiatry.cam.ac.uk/software/; Cambridge, UK) using a restricted search volume mask of the orbitofrontal cortex taken from Hammer’s probabilistic atlas (Hammers et al., 2003). Body mass index values for each participant were regressed onto the grey matter volume maps, searching for voxels that were significantly associated with BMI. Years of cocaine use were similarly regressed on grey matter maps in cocaine-dependent individuals. Association was determined using clusterlevel spatial permutation testing (Suckling & Bullmore, 2004), and significance levels were set at p~0.0002, controlling for multiple comparisons. These results were exported to 127 Chapter 6. OFC loss with BMI and cocaine use Statistical Package for Social Sciences (SPSS v.19) and compared post-hoc using Pearson correlation coefficients and independent samples t-tests for group comparisons of grey matter volume loss; significance levels were set at p<0.05. 3. Results In control participants four significant clusters negatively correlated with BMI bilaterally throughout the OFC (r=-0.688, p<0.001; Figures 6.1 and 6.2), while in cocaine users two regions in the right OFC negatively correlated with BMI (r=-0.624, p<0.001; see Appendix D, Table S6.1 for coordinates). These areas overlapped in the right middle orbitofrontal gyrus, superior orbitofrontal gyrus and gyrus rectus (Brodmann Areas 10, 11, 25). Additionally, OFC volume negatively correlated with years of cocaine use in cocainedependent individuals in two bilateral clusters (r=-0.705, p<0.001). The cocaine-related decline in grey matter overlapped with BMI-related reductions bilaterally in the superior orbitofrontal gyrus, middle orbitofrontal gyrus and left gyrus rectus (Brodmann Area 11). 128 Chapter 6. OFC loss with BMI and cocaine use -28 -24 -20 -16 -12 -8 Figure 6.1 Overlapping regions of orbitofrontal cortex (OFC) grey matter volume decline among healthy control and cocaine-dependent individuals, corresponding to increased body mass index (BMI) and years of cocaine use. Magenta – clusters for reduced grey matter with BMI in controls; Blue – clusters for reduced grey matter with BMI in cocaine users; Green – clusters for reduced grey matter with years of cocaine use; Cyan – overlapping voxels from magenta and green. 129 Chapter 6. OFC loss with BMI and cocaine use Grey matter volume related to body mass index A) Grey matter volume related to years of use B) Figure 6.2. A) Negative correlation between OFC grey matter volume and BMI in control and cocaine-dependent individuals. Groups were analysed separately and then overlaid to demonstrate similar decreases in OFC volume in association with increased BMI. B) Correlation between OFC volume reductions and years of cocaine use. 130 Chapter 6. OFC loss with BMI and cocaine use In cocaine users, we observed a significant correlation between grey matter volume decline associated with increased BMI and with years of cocaine use (r=0.354, p=0.006; Figure 6.3); however, cocaine use and BMI itself did not correlate significantly (r=-0.127, p=0.338). This suggests an overlapping reduction related to both increased BMI and prolonged cocaine use, their effects on the brain potentially impacting the same regions. Figure 6.3. Correlation between OFC grey matter volume associated with years of cocaine use and OFC volume related to BMI in cocaine-dependent individuals. As there was no direct correlation between years of cocaine use and BMI, a significant positive correlation suggests there is a relationship between the effect these two variables (years of cocaine use and BMI) have on the OFC independently of one another. 131 Chapter 6. OFC loss with BMI and cocaine use When directly comparing total OFC volume between the two groups, cocaine-dependent individuals had significantly less grey matter than controls (t=7.694, p<0.001). This effect remained significant after including only overweight controls (n=34) and lean cocainedependents (n=37; t=4.097, p<0.001). However, the steepness of the linear regressions between the key variables (grey matter and BMI, and grey matter and cocaine years) did not significantly differ. This suggests that while the most pronounced effects on orbitofrontal structure were associated with cocaine use, this is perhaps more indicative of premorbid differences rather than an accumulated effect of stimulant use, though other studies have shown evidence to the contrary (Ersche, Williams, Robbins, & Bullmore, 2013; Porrino et al., 2007). 4. Discussion Years of cocaine use and increased BMI both negatively correlated with grey matter volume in the OFC in healthy control and cocaine-dependent individuals. Additionally, select OFC regions showed decreases relating to both variables, suggesting an overlap between volume reductions associated with over-consumption of food and drugs of abuse. This decline may reflect underlying differences in the OFC’s role in goal-directed behaviour present in both groups, as both begin as motivated behaviours (taking drugs or eating high-caloric foods for hedonic reasons), but in some instances can become compulsive, habitually maintained after the behaviour is no longer desired (Balleine & O’Doherty, 2010). Both cues for high-caloric foods and drugs of abuse activate similar regions in dependent drug users and obese individuals (Tang, Fellows, Small, & Dagher, 2012), and the OFC is activated in terms of reward responding to multiple types of stimuli, including monetary reward, food and drugs of abuse during experiences of craving (FitzGerald, Seymour, & Dolan, 2009; Garavan et al., 132 Chapter 6. OFC loss with BMI and cocaine use 2000; Kim, Shimojo, & O’Doherty, 2011; Levy & Glimcher, 2012). Associated impairments in inhibitory control and decision-making may also be implicated as consequences of OFC volume reduction. It should be noted that while many studies show similar BMI-related declines in grey matter volume, other investigations report no differences in brain structure associated with obesity. For example, one longitudinal study of middle-aged to elderly adults found no additional changes in grey matter volume associated with an increase in BMI (Driscoll et al., 2012). Another investigation actually reported an increase in grey matter in the medial orbitofrontal cortex and ventral striatum in obese individuals (Horstmann et al., 2011). Other research has suggested that the changes seen in grey matter volume are related to fat-free mass, rather than body fat percentage (Weise, Thiyyagura, Reiman, Chen, & Krakoff, 2013). There are also notable gender differences seen in prior research on the topic, with some studies showing associations between BMI and brain volume only in male participants (Taki et al., 2008), while other investigations report these results solely in women (Walther et al., 2010). Finally, many of these studies have been conducted in elderly individuals, particularly those already showing evidence of cognitive impairment or Alzheimer’s disease (Ho et al., 2010; Raji et al., 2010). This is a confounding variable and suggests that the effect of BMI on the brain may be greatest in patients already experiencing cognitive and structural decline; however, recent investigations into obese children and adolescents have also reported similar grey matter volume decline (Alosco et al., 2014; Yokum, Ng, & Stice, 2012). Thus, with the recent proliferation of research into the topic, there are conflicting reports regarding the extent and potential mechanisms behind these findings, and more research is needed on the topic to better examine these findings. 133 Chapter 6. OFC loss with BMI and cocaine use Importantly, the participants in the current study were not recruited on the basis of their BMIs, and their range of normal weight to obese was representative of the general population. They were also healthy individuals without any pre-existing physical, psychological or cognitive impairments. This demonstrates that the relationship with grey matter volume is present even in persons of normal or slightly elevated body weight, and is not limited to obese individuals. While these findings provide some support for the argument that some individuals who overeat may display characteristics similar to those of addictive disorders, this comparison does have its limitations and is currently under debate (Ziauddeen et al., 2012). 134 Chapter 7. Discussion Chapter 7. General Discussion 1. Summary The research presented in this thesis provides evidence for both neurocognitive risk and protective factors involved in addictive disorders, focusing on the conditions of stimulant dependence and obesity resulting from binge eating behaviour. These include differences in inhibitory control and reward valuation in the face of both general and substance-specific stimuli. Behavioural abnormalities also corresponded to variations in structure and function in regions of the brain implicated in cognitive control and reward processing, particularly in areas of the prefrontal cortex, such as the inferior frontal gyrus (IFG) and medial orbitofrontal cortex (OFC). The first half of this thesis centred on stimulant addiction, researching both dependent and recreational users of cocaine, as well as the non-dependent biological siblings of addicted individuals using endophenotype research. These studies were conducted in an attempt to identify underlying risk factors for dependence, compared with more long-term effects of drugs on the brain and behaviour, as well as potential protective or resilience traits against its development. This research was aimed at answering the chicken-or-egg question of which abnormalities typically seen in cases of substance dependence are predating neurocognitive differences that can place an individual at a heightened risk for dependence, and which are the result of chronic drug use. The study presented in Chapter 2 investigated executive control and correlative neural activation in stimulant-dependent individuals (SDIs), their non-dependent biological siblings 135 Chapter 7. Discussion and unrelated healthy controls using the classic colour-word Stroop task. A decline in cognitive efficiency resulting in significantly slower global response times and relating to decreased volume in the IFG was seen in both SDIs and their siblings, suggesting that these impairments are not the result of drug abuse but are instead underlying risk factors for addiction. However, there was an additional decrease in cortical volume in the dependent individuals, indicating a potential exacerbating effect of stimulant use on this pre-existing trait. The lack of difference in activation during the task between SDIs and controls was surprising, particularly when compared with the significant decrease in activity seen in the sibling participants. One possible explanation is that the SDIs may have potentially initially experienced decreases in activation similar to their non-dependent siblings, but that either the acute or the chronic effects of stimulant drugs on the brain have affected their neural responding. This would imply that the decreased IFG activation was, in fact, an endophenotype of dependence, perhaps reflecting poor cognitive efficiency. However, this interpretation is speculative and should be taken with caution. In Chapter 3, a study of dependent and recreational stimulant users and healthy control volunteers revealed that stimulant-dependent individuals were significantly more impaired on the cocaine-word Stroop – a task of cognitive inhibition involving cocaine cues – demonstrating significant attentional bias to the salient cocaine words, while recreational users showed no signs of impairment on the task. Additionally, recreational individuals displayed significantly different patterns of activation during performance, with a decrease in prefrontal and orbitofrontal cortical activity compared with SDIs. One possible interpretation for these findings is that cognitive control was more efficient in recreational users, with 136 Chapter 7. Discussion resistance to distraction by cocaine cues being less effortful in these individuals. Meanwhile in the SDIs, performance of the task may have required greater recruitment of neural resources, as the objective was significantly more difficult to execute. The relative decrease in OFC activity in the recreational users could also be linked to an absence of craving in these individuals. The OFC has been implicated in reward valuation and motivation for a stimulus (O’Doherty, 2002), and craving for cocaine cues has been linked to increased OFC activity (London et al., 2000; Wang et al., 1999). A higher value placed upon cocaine for dependent users could result in greater activation in this region, and activity in the area has been correlated with self-report craving scores in dependent users (Grant et al., 1996; London et al., 2000; Volkow et al., 1991). The relative decrease in OFC activity in recreational users suggests they are not experiencing cue-induced craving for cocaine in a similar manner as the SDIs and instead may have some resilience against these types of addictive behaviours. Decision-making abilities have also been linked to the OFC, with patients with ventromedial prefrontal cortex lesions exhibiting severe impairment on a decision-making task (Bechara et al., 1994). Dependent drug users have shown similar dysfunction on decision-making assessments, potentially due to drug-induced abnormalities in this region (Bechara et al., 2000; Ersche et al., 2005). The under-activation of recreational users in this area, combined with larger structural volumes, suggests that they may exert greater self-control and demonstrate improved decision-making abilities, particularly compared to dependent individuals. This could be particularly applicable in situations involving ‘myopia for the future’ where long-term consequences must be weighed against short-term rewards. Drug users are typically impaired when making these types of decisions (Kirby, Petry, & Bickel, 137 Chapter 7. Discussion 1999), particularly when drug use is involved. However, the recreational users’ anecdotal ability to prioritize work or school above drug taking suggests they may have improved selfcontrol and future planning. Finally, the OFC is commonly implicated in habitual repetitive behaviours characteristic of obsessive-compulsive disorder (OCD), and impairment in this region in drug-dependent individuals is thought to contribute to the compulsive drug-seeking and taking behaviours present in addiction (London et al., 2000). Several studies investigating patients with OCD have demonstrated an increase in glucose metabolism in the OFC, indicating hyperactivity in this region (Baxter et al., 1988; Menzies et al., 2008). The contrasting decrease in activity in the recreational users in this area may act as a potential protective factor against compulsive behaviours (Ersche, Jones, Williams, Smith, et al., 2013), thus making them less likely to develop drug dependence. Building on these findings, in Chapter 4, we investigated all four participant groups – SDIs, their non-dependent biological siblings, recreational users of cocaine and unrelated healthy control volunteers – on a separate neurobiological construct, assessing differences in responding to both universal (money) and drug-specific reward cues. We examined how reactions to these two stimuli related to both stimulant use and a risk for dependence. Rather than finding a potential endophenotype for dependence, as reported in previous investigations with these groups (Ersche, Jones, et al., 2012; Ersche, Turton, et al., 2012), the results of the current study pointed towards consequential effects of drug exposure on brain responses to reward, particularly in the OFC and striatum. Both SDIs and recreational users displayed increased BOLD activation in response to drug cues compared with the sibling and control participants; however, there were additional increases in striatal activity in the SDIs that were 138 Chapter 7. Discussion not present in the recreational users. This may be indicative of an increased exposure effect in the SDIs resulting in greater anticipatory excitation, reflective of the findings presented in Chapter 3 of greater attentional bias to cocaine cues. Curiously, there was a difference between the recreational and dependent individuals in the area that was most activated in response to anticipation for drug reward. The SDIs showed a greater response in the striatum compared with all the other participant groups, including the recreational individuals, whereas the recreational users showed the greatest difference in the OFC. This may be suggestive of a difference between the quality of drug use between the two groups, or this may be representative of the difference in exposure between these participants. One possibility is that increased activation in the striatum in response to drug cues is reflective of greater compulsive-like tendencies in regards to drug taking. The putamen is strongly implicated in habit formation (Yin & Knowlton, 2006), and SDIs showed significantly increased activation in this area in response to anticipation for drug reward, suggesting they have a compulsive or habitual response to these stimuli that is not present in the recreational cocaine users. Conversely, activation in the OFC in response to drug cues may be reflecting an evaluative response to these stimuli (Levy & Glimcher, 2012), which both groups of drug users would presumably rate more highly. In addition to the differences in responding to drug cues, there was also an exposure effect in regard to monetary rewards. The sibling and control individuals displayed greater activation in the OFC in response to successful feedback on the money trials compared with both dependent and recreational users. This suggests there may be a partial flattening to non-drug rewards in the stimulant users, potentially reflective of the reward deficiency theory of addiction, with a ‘hijacking’ of the reward system by drugs of abuse. However, this exposure 139 Chapter 7. Discussion effect was only present in response to monetary reward receipt, not anticipation. This is perhaps suggestive of the incentive salience theory of reward processing (Robinson & Berridge, 1993), with anticipatory cues becoming ‘more valuable’ and eliciting a heightened response compared with obtaining the reward itself. One potential explanation for these differences in sensitivity to reward is an alteration in dopamine receptor density in the mesolimbic system. A decrease in dopamine receptor availability has been linked to a greater proclivity for stimulant drug use, with individuals with fewer dopamine D2 receptors finding the acute effects of stimulants more enjoyable, and thus reinforcing repeated use (Volkow et al., 1996, 1999). A previous investigation into the relatives of alcohol-dependent individuals showed an increase in striatal dopamine receptor density compared with control individuals without a family history of dependence (Volkow et al., 2006). Thus it is possible that the sibling individuals in the current study have a relative increase in striatal dopamine receptor density compared with their dependent brothers and sisters, protecting them potential reinforcing properties of drugs of abuse. However, we did not see any increases in striatal responding in the sibling participants, so there does not appear to be support for this theory in the current findings. In the second half of this thesis, research was extended to include an additional group of individuals who, it has been argued, also fall within the spectrum of addictive disorders – obese individuals with binge eating behaviours (Davis & Carter, 2009; Ifland et al., 2009; Parylak, Koob, & Zorrilla, 2011; Pelchat, 2002). This cohort, who show some of the same behavioural and neurobiological tendencies as individuals with substance dependence (Avena et al., 2008; Volkow, Wang, Fowler, et al., 2008; Wang et al., 2004), was considered as a potential comparison group for dependent stimulant users, as they maintain similar traits of 140 Chapter 7. Discussion impulsivity, compulsivity and decreased executive function, but do not have any of the additional changes in the fronto-striatal dopamine system caused by the use of stimulant drugs. Thus, this group could potentially provide further insight into possible shared underlying risk traits for maladaptive addictive behaviours and help to distinguish them from the consequences of long-term drug use on the brain. In Chapter 5, responding to cues of reward was again investigated, this time with a novel group of cocaine-dependent men, as well as obese individuals with binge eating behaviours (OBEs). Behavioural responses to a variety of rewarding stimuli were studied to provide a more thorough investigation of differences in sensitivity to both generic and substancespecific reinforcers. This enabled us to address the two theories of disordered reward responding in substance-dependent individuals: an overall under-active reward system causing drugs of abuse to be deemed disproportionately reinforcing (Blum et al., 2000), or a general increase in responsivity to rewarding stimuli, which is then transferred particularly to drug-related cues and can hijack this system (Robinson & Berridge, 1993). There were few significant differences in response times both between and within participant groups to the different stimuli. Overall, the OBE participants trended towards displaying slower reactions to the cues, particularly in response to food stimuli. Conversely, the SDIs showed quicker response times, especially for the money cues. From these results, rather than sharing similar abnormalities in reward processing systems, it appears that the two addiction spectrum groups might each potentially fulfil one of the different theories on dysfunctional reward valuation. The overall depressed responding to reward cues in the OBE group is suggestive of the reward deficiency theory (Bjork, Smith, & Hommer, 2008; Blum et al., 2000; Blum, Cull, Braverman, & Comings, 1996), which has been proposed previously to 141 Chapter 7. Discussion explain some of the behavioural traits specifically involved in obesity (Davis & Fox, 2008; Davis, Strachan, & Berkson, 2004). Conversely, based on results from Chapters 4 and 5, it appears that stimulant-dependent individuals adhere more to the incentive sensitization theory, displaying heightened sensitivity to several types of rewarding stimuli, including those associated with drug use (such as stimulants and potentially monetary rewards) as well as more general reinforcers. However, it should be cautioned that the results from Chapter 5 are only suggestive, and significant demographic differences between the groups, particularly age and gender, may have accounted for the group effects. This included an overall increase in age in the OBE group compared both to SDIs and controls, which was strongly associated with globalized slower responding on the task. In Chapter 6, these group comparisons were further explored by investigating how structural differences in the key region of the orbitofrontal cortex – implicated in reward valuation and decision-making – were associated with both cocaine use and body mass index (BMI). A decline in OFC volume was associated with years of stimulant use, as reported in previous investigations (Ersche et al., 2011; Franklin et al., 2002; Matochik et al., 2003; Sim et al., 2007), as well as an increase in BMI in both cocaine-dependent men and healthy control volunteers. Moreover, there was an overlap in the regions and specific voxels in which declines were seen both between participant groups and associated with each condition. This finding is the first to identify a specific overlap in areas affected by stimulant use and weight gain, which potentially provides fodder for the argument of overeating leading to obesity in some instances qualifying as an addictive disorder. However, it should be noted that an overall greater decline was seen in cocaine-dependent men compared to the healthy 142 Chapter 7. Discussion control volunteers, regardless of their BMI. This indicates that although there was an overlap in brain volume trends between the two conditions, greater structural dysfunction was associated with stimulant use. While a decrease in grey matter volume has been shown to directly result from drug use (Porrino et al., 2004, 2007), and despite the several studies showing a decrease in structural integrity in the brains of obese individuals (Gunstad et al., 2008; Maayan, Hoogendoorn, Sweat, & Convit, 2011; Pannacciulli et al., 2006; Taki et al., 2008; Walther et al., 2010), it is as yet unknown whether these differences are caused by an increase in BMI or are underlying abnormalities contributing to the condition. It is possible that predating decreases in orbitofrontal volume in both groups led to a pattern of behaviour associated with heightened risk for addictive behaviours, such as low self-control or a greater tendency towards habit formation. Additionally, evidence of cognitive disruption in obese individuals, which would be hypothesised to mirror that of substance-dependent individuals given some of their similar structural abnormalities, has been conflicting. Some studies provided evidence for deficits in executive functioning in obese individuals, such as decreased working memory or decisionmaking abilities; however, other investigations have shown no such dysfunction, or even an improvement in performance on other cognitive constructs (Elias, Elias, Sullivan, Wolf, & D’Agostino, 2003; Gunstad et al., 2008; Gunstad, Lhotsky, Wendell, Ferrucci, & Zonderman, 2010; Stanek et al., 2013). Thus, the functional impact of these structural differences, as well as their origin, is still unknown. 2. Links to Personality Traits Other research that has been referred to throughout this dissertation, and that I have contributed to, reports on key personality traits involved in addiction in the four participant 143 Chapter 7. Discussion groups examined in Chapters 2-4 (Ersche, Jones, Williams, Smith, et al., 2013). This paper is included as Appendix E. Briefly, it was discovered that the SDIs and their biological siblings had significantly higher rates of impulsivity and compulsivity than healthy control volunteers, suggesting that these personality traits may serve as endophenotypes for stimulant dependence. However, there was an additional increase in these qualities in the SDIs compared with their siblings, implying an additional drug effect on these characteristics. Conversely, sensation-seeking scores were elevated only in the SDIs and recreational users of cocaine, suggesting that this trait may correspond to an increased likelihood to initiate drug use. Recreational users were no different in rates of impulsivity and compulsivity than control volunteers, potentially protecting them from transitioning to drug dependence (Figure 7.1). These findings led us to believe that impulsivity and compulsivity are endophenotypic traits, acting as underlying risk factors for stimulant dependence. Conversely, sensation-seeking appears to be specific to the initiation of drug use, possibly representing a motivating quality to first seek out experiences with stimulant drugs. The decrease in impulsive and compulsive traits in the recreational users may have protected them from the development of dependence from their casual drug-taking, while low sensation-seeking may have prevented the biological siblings of dependent drug users from ever initiating drug use, thus protecting them against the potential increased likelihood for a development of dependence had they started taking drugs. 144 Chapter 7. Discussion Figure 7.1. Group differences in the personality traits of impulsivity, compulsivity and sensation-seeking in the four groups of stimulant-dependent individuals, their non-dependent biological siblings, recreational users of cocaine, and unrelated control volunteers, as reported on in Chapters 2-4. Reproduced from (Ersche, Jones, Williams, Smith, et al., 2013). 145 Chapter 7. Discussion These personality traits were also linked to the behavioural and neurobiological characteristics examined in this dissertation. Decreases in grey matter volume in the inferior frontal gyrus, as reported in Chapter 2, was associated with an increase cocaine-specific compulsivity as measured by the OCDUS (right IFG: r=-0.297, p=0.056; left IFG: r=-0.308, p=0.047), as well as compulsivity scores on the PADUA (Burns, Keortge, Formea, & Sternberger, 1996), an assessment of general obsessive-compulsive behavioural traits (right IFG: r=-0.237, p=0.05; left IFG: r=-0.297, p<0.01). Impulsivity, as measured by the BIS-11, correlated with response times on the congruent condition of the colour-word Stroop (r=0.249, p=0.003); and scores on the PADUA, BIS-11 and SSS-V sensation-seeking scale all negatively correlated with activation in the right IFG during the group contrast on the colour-word Stroop (r=-0.228, p=0.007; r=-0.271, p=0.001; r=-0.196, p=0.022, respectively), such that higher scores on these measures was associated with a decrease in right IFG activation on the task. On the cocaine Stroop task, BIS-11 impulsivity scores were positively correlated with response times on the cocaine word trials (r=0.280, p=0.002), as well as with interference scores (r=0.193, p=0.040) and errors committed on the task (r=0.344, p<0.001). Impulsivity scores were also correlated with activation during the cocaine Stroop task in the orbitofrontal cortex/anterior cingulate cortex (r=0.218, p=0.019) and two clusters in the inferior frontal gyrus (r=0.220, p=0.018; r=0.277, p=0.003). Sensation-seeking scores did not relate to behavioural performance on the task, but there was a significant negative correlation between sensation-seeking and activation in the right angular gyrus during the group performance comparison (r=-0.205, p=0.028). 146 Chapter 7. Discussion On the Monetary Incentive Delay task, anticipatory activation for the drug rewards in the left middle cingulum significantly correlated with impulsivity and sensation-seeking (r=0.210, p=0.013; r=0.213, p=0.011, respectively). BIS-11 impulsivity scores also related to increased activity in the striatum during anticipation for drug rewards (r=0.174, p=0.040). Behaviourally, there were no correlations between personality traits and performance on the task, except for an increase in money won on the task relating to increased impulsivity (r=0.260, p=0.002) and OCDUS cocaine compulsivity scores (r=0.272, p=0.040). From this research it appears there are three crucial components in the development of substance dependence: sensation-seeking and high reward sensitivity to facilitate and spur initial experimentation with the drug; impulsivity and poor cognitive control resulting in the continued and escalated use of the substance, despite potentially disadvantageous consequences or a desire not to; and following from that, compulsivity leading to habitual use of the drug even in the face of punishment, attempts to stop, and loss of the acutely rewarding sensations first experienced. Accordingly, stimulant-dependent individuals met all of these criteria, with high levels of sensation-seeking, impulsivity and compulsivity, as well as low cognitive control. Their non-dependent biological siblings shared similar levels of impulsivity, compulsivity and poor self-control, but crucially did not demonstrate the sensitivity to reward or sensation-seeking traits to cause them to take the first step and initiate drug use. Conversely, recreational users of cocaine had high levels of sensation-seeking and in similar tendencies towards reward valuation as the dependent individuals, particularly during anticipation for drug rewards. However, these individuals lacked the critical increases in impulsivity and compulsivity implicated in dependence, and had much higher levels of cognitive control compared with the dependent individuals and their siblings, and even in some instances compared with healthy control participants. 147 Chapter 7. Discussion These patterns of behaviour appear to be stable traits in these participant groups, manifested in several different types of behavioural and neurobiological assessments, including a neutral cognitive control test (colour-word Stroop), an assessment of attentional bias and distraction to cocaine stimuli (cocaine-word Stroop), and responsivity to both general and drug-related reward cues (Monetary Incentive Delay). Thus, these tendencies seem to be robust findings strongly suggestive of predating abnormalities in the brains and behaviours of individuals who maintain a greater risk for stimulant dependence, as well as crucial unique protective factors in those who have remained resilient to it. 3. Synthesis Despite the evidence for endophenotypic traits present in both the dependent participants and their biological siblings, there were also additional changes in the brains of dependent individuals compared to their siblings. These differences may have been the result of chronic stimulant use on the brain, though they were largely unseen in the recreational users, or they could represent additional deficits that placed these individuals at a heightened risk for dependence. Conversely, the additional differences in the recreational and sibling participants could be protective traits that were present in these individuals and kept them safe from the development of drug dependence, either preventing initial use or enabling them to maintain control over their behaviours if they did take drugs. In the studies involving the recreational participants, the finding of critical differences between recreational and dependent users of cocaine, including a lack of distraction, emotional salience and sensitivity to reward in the face of cocaine-related stimuli, indicates that there may be inherent behavioural and neurobiological differences between those who 148 Chapter 7. Discussion can use a class A drug such as cocaine recreationally without making the transition to dependence, and those who have become dependent upon the substance. In the second half the thesis, a separate cohort of individuals potentially on the addiction spectrum – obese individuals with binge eating behaviours – was investigated for similar traits of reward valuation and orbitofrontal cortex functioning as those of stimulantdependent individuals. However, in the few tests conducted on these participants, there was only a small overlap in their characteristics compared to the stimulant-dependent individuals. Instead, the greatest similarity appeared to be between OBEs and the sibling participants tested in the earlier studies. This included tendencies towards impulsive behaviours, but no increase in sensation-seeking, as well as flattened reward responding to both general and specific reinforcers. For example, while these participant groups were not tested on the same battery and were not initially planned on being compared, it is interesting to note that in posthoc analyses, the sibling and OBE participants did not differ on self-report measures of impulsivity using the BIS-11, but both groups were significantly higher than control and recreational participants on this measure (see Figure 7.2). Additionally, both groups also showed lower sensation-seeking scores than the dependent and recreational users, with the OBE participants scoring even lower than the controls and sibling participants. Thus, these obese individuals appear to maintain impulsive traits potentially placing them at a higher risk for addictive tendencies, but not the reward and sensation-seeking characteristics that would incite them to initiate drug use. Instead, their maladaptive patterns of behaviour manifested onto the more traditional and omnipresent reinforcer of high-caloric foods. 149 Chapter 7. Discussion Figure 7.2. Group differences in impulsivity (BIS-11) and sensation-seeking (SSS-V) scores between control participants, obese individuals with binge-eating behaviours, recreational cocaine users, dependent stimulant users and their non-dependent biological siblings. Obese binge-eating individuals were most similar to the sibling 150 Chapter 7. Discussion participants, with impulsivity scores higher than controls and recreational users, but lower than dependent individuals. On a measure of sensation-seeking, obese individuals expressed the lowest scores, most similar to sibling and control participants. This provides evidence for a strong similarity between obese individuals with binge-eating tendencies and the siblings of stimulant-dependent individuals, possibly showing attributes suggestive of dependent behaviours, but without the high sensation-seeking that is most strongly linked to the initiation of drug experimentation. Thus, these individuals’ addictive tendencies may manifest in a different manner or to another type of substance, like food, rather than through drug use. Personality measures taken from. Several genetic links have been explored to potentially explain some of the overlap between addictive and eating disorders. This has primarily focused on differences in genes, such as TaqIA, involved in dopamine transmission due to the neurotransmitter’s role in motivated behaviours and reward system functioning (Blum et al., 1990; Comings et al., 1993; Comings, Muhleman, Ahn, Gysin, & Flanagan, 1994; Davis et al., 2009; Felsted, Ren, Chouinard-Decorte, & Small, 2010; Jonsson et al., 1999; Noble, 2000; Noble et al., 1993; Stice, Spoor, Bohon, & Small, 2008). Specifically, alleles resulting in reduced dopamine receptor density or decreased dopamine transporter availability have been linked to a greater risk for impulsive disorders and an increase in both the expected and experienced pleasure derived from rewarding substances, such as high-caloric foods or drugs of abuse (Comings et al., 1993, 1994; Felsted et al., 2010; Spitz et al., 2000; Stice et al., 2008; Thanos et al., 2001). An alternative genetic explanation involves the monoamine oxidase A (MAOA) gene, which is implicated in the metabolism of monoamine neurotransmitters, subsequently affecting cerebral levels of dopamine, noradrenaline and serotonin (Ducci et al., 2006). A 151 Chapter 7. Discussion polymorphism in the gene transcription results in a decrease in monoamine oxidase A activity, reducing the turnover of monoamines and thus leading to an increase of these neurotransmitters in the synapse. This sequencing has subsequently been associated with heightened aggression, impulsivity and increased risk for addiction (Caspi et al., 2002; Philibert, Gunter, Beach, Brody, & Madan, 2008; Saito et al., 2002), although other investigations have found no such link between the polymorphism and these behaviours (Koller, Bondy, Preuss, Bottlender, & Soyka, 2003). Due to dopamine’s known involvement in eating behaviours and reward valuation (Baldo & Kelley, 2007; Wang et al., 2001), the MAOA gene has also been investigated as a potential factor in over-eating leading to obesity (Camarena et al., 2004; Ducci et al., 2006). As such, the presence of the polymorphism resulting in low MAOA activity was found to be associated with elevated dopamine metabolites and an increase in body weight and body mass index among both alcoholdependent and healthy control individuals (Ducci et al., 2006). Finally, the MAOA gene is also implicated in cortical volume, particularly in the OFC, and presence of the polymorphism resulting in low monoamine oxidase A activity has been linked to decreased functioning in this area (Meyer-Lindenberg et al., 2006). Furthermore, when combined with stimulant use, presence of the low MAOA expression type can lead to a significant exacerbation of the effects of stimulant drugs on cortical volume, resulting in escalated grey matter decline, particularly in the OFC (Alia-Klein et al., 2011). Thus, there is a potential genetic link between elevated BMI, substance dependence and decline in OFC grey matter volume, as presented in the findings reported in Chapter 6. 152 Chapter 7. Discussion 4. Future Directions A primary concern regarding the investigations presented in this thesis is the ranging differences in demographics between the participant groups. These include age, gender distribution, educational attainment, and the co-morbid presence of depression and alcohol abuse in the SDIs. Although we attempted to control for these variables in statistical analyses, better matching for these variables and other demographics during recruitment could be improved in future studies. However, it should be noted that some of these demographic differences might be intrinsically linked to the participant groups, such as higher education acting as a protective factor in recreational users, or the presence of polysubstance abuse in the dependent individuals. Another possible point of contention in these investigations is that ‘recreational cocaine use’ is not a precisely defined categorization. In the studies presented in this thesis, criteria based on the quantity and quality of use, as well as an absence of any family history of substance dependence, were used to make these classifications. However, research from other laboratories has focused solely on the quantity and frequency of cocaine used when making their evaluations. As such, previous investigations have included individuals who our classifications would have designated as ‘abusive’ rather than ‘recreational’ users. These discrepancies in inclusion criteria may help to explain the difference in findings reported here in comparison to prior studies of non-dependent stimulant users (Colzato et al., 2007; Soar et al., 2012). A universal definition for this type of use would be highly beneficial when making these sorts of distinctions between recreational, abusive and dependent behaviours in future research. 153 Chapter 7. Discussion The relatively short abstinence period of the stimulant-dependent individuals should also be better controlled for, with a minimum abstinence requirement to ensure results are not effects of either acute stimulant use or withdrawal, but are stable traits of this group. Subsequent studies could employ genetic analyses to elucidate whether similarities in performance between the stimulant-dependent and sibling groups were due to inherited traits or shared environmental experiences during development. As the sibling pairs were raised in the same households, it is difficult to discern the extent to which environmental effects, such as low socioeconomic status, might have affected these individuals. These experiences could cause them to differ in performance from control and recreational participants, who were less likely to be exposed to these challenges growing up. Conversely, inherited polymorphisms involving the TaqAI or MAOA genes, among others, may have contributed to their differences in performance and heightened risk for dependence. Future investigations should use positron emission tomography (PET) to measure dopamine receptor availability and binding, particularly in recreational users of cocaine and the nondependent siblings of addicted individuals, given the neurotransmitter’s known involvement in addictive disorders. This would be particularly informative in regards to aberrations in reward sensitivity, as stated in Chapter 4, and could help provide a neurobiological explanation for some of the behavioural and functional differences seen between these participant groups. In order to determine other potential similarities between substance dependence and binge eating disorder, assessments traditionally used to measure impairments associated with stimulant dependence – such as impulsivity using the Stop Signal Reaction Time task, or the Monetary Incentive Delay task as a more standardised assessment of reward sensitivity – 154 Chapter 7. Discussion should be administered. Additionally, an assessment of attentional bias to food-related stimuli, such as a food-related Stroop task, should be implemented. Another assessment, the delay discounting task, could also be used to measure reward-related impulsivity to both general (money) and substance-specific (food or drugs) stimuli. 5. Conclusions and Implications The chicken-or-egg debate of substance dependence has dominated much of the field of addiction research over the last decade – whether behavioural and neurobiological abnormalities commonly seen in drug-dependent individuals are underlying traits that foster an increased vulnerability for dependence, or if they are the consequence of long-term use. Here, evidence is presented regarding predating risk factors that can place an individual at a greater susceptibility for experimentation with illicit substances, as well as an additional heightened risk for the subsequent development of dependence. However, evidence is also presented for resilience traits that can potentially protect against the initiation of drug use, as well as the slide into addiction. The findings presented in this thesis provide support for the notion that drug addiction is an underlying neurobiological disorder, rather than a volitional choice made by those who are dependent on a substance (Nutt, 2013). These studies could be used as evidence in the argument for using rehabilitative treatments rather than more draconian tactics when dealing with drug-related crimes. This research could also help in the development of addiction prevention programs that are specifically targeted towards individuals who maintain a higher risk for dependence. By identifying these individuals earlier on – i.e. during adolescence and before they have begun experimenting with drugs – and with simple and easily administered questionnaires or neurocognitive tests – more focused prevention or harm reduction methods 155 Chapter 7. Discussion could be implemented. Additionally, the protective factors identified in the sibling and recreational participants, particularly of decreased reward valuation, could be utilized to encourage those at risk to indulge in other sorts of less harmful rewarding stimuli instead of drugs of abuse or over-eating. Finally, while there was only marginal support for the classification of binge eating leading to obesity as an addictive disorder, the findings from these investigations could still be used to identify and target those individuals who might be at a greater susceptibility to this type of behaviour as well. This also includes those individuals with cognitive control difficulties, increases in impulsivity, and potentially greater tendencies towards habitual or compulsive behaviour stemming from abnormalities in the orbitofrontal cortex, but without the key trait of sensation-seeking. While these studies cannot yet answer the question of how to prevent unhealthy dependencies, they can help bring us one step closer to identifying and potentially treating those who are at the highest risk for addiction. 156 References References Aguilar de Arcos, F., Verdejo-García, A., Ceverino, A., Montañez-Pareja, M., LópezJuárez, E., Sánchez-Barrera, M., … Pérez-García, M. (2008). Dysregulation of emotional response in current and abstinent heroin users: Negative heightening and positive blunting. Psychopharmacology, 198(2), 159–166. Aguilar de Arcos, F., Verdejo-Garcia, A., Peralta-Ramirez, M. I., Sanchez-Barrera, M., & Perez-Garcia, M. (2005). Experience of emotions in substance abusers exposed to images containing neutral, positive, and negative affective stimuli. Drug and Alcohol Dependence, 78(2), 159–167. Aigner, T., & Balster, R. (1978). Choice behavior in rhesus monkeys: cocaine versus food. Science, 201(4355), 534–535. Aitken, C., Kerger, M., & Crofts, N. (2000). Drivers who use illicit drugs: behaviour and perceived risks. Drugs: Education, Prevention, and Policy, 7(1), 39–50. Albery, I. P., Strang, J., Gossop, M., & Griffiths, P. (2000). Illicit drugs and driving: prevalence, beliefs and accident involvement among a cohort of current out-oftreatment drug users. Drug and Alcohol Dependence, 58(1-2), 197–204. Alia-Klein, N., Parvaz, M. A., Woicik, P. A., Konova, A. B., Maloney, T., Shumay, E., … Goldstein, R. Z. (2011). Gene x disease interaction on orbitofrontal gray matter in cocaine addiction. Archives of General Psychiatry, 68(3), 283–294. Alosco, M. L., Stanek, K. M., Galioto, R., Korgaonkar, M. S., Grieve, S. M., Brickman, A. M., … Gunstad, J. (2014). Body mass index and brain structure in healthy children and adolescents. The International Journal of Neuroscience, 124(1), 49–55. American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders (4th ed.). Washington, D.C.: American Psychiatric Association. Andersson, J., Jenkinson, M., & Smith, S. (2007a). Non-linear optimisation. In FMRIB technical report TR07JA1. Andersson, J., Jenkinson, M., & Smith, S. (2007b). Non-linear registration, aka Spatial normalisation. In FMRIB technical report TR07JA2. Asensio, S., Romero, M. J., Palau, C., Sanchez, A., Senabre, I., Morales, J. L., … Romero, F. J. (2010). Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study. Addiction Biology, 15(4), 504–516. Avena, N. M., Rada, P., Bocarsly, M., & Hoebel, B. G. (2005). Sugar bingeing produces effects on opioid and dopamine systems: Possible roles in drug abuse. Neuropsychopharmacology, 30, S30–S30. 157 References Avena, N. M., Rada, P., & Hoebel, B. G. (2008). Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience and Biobehavioral Review, 32(1), 20–39. Avena, N. M., Rada, P., & Hoebel, B. G. (2009). Sugar and fat bingeing have notable differences in addictive-like behavior. Journal of Nutrition, 139(3), 623–628. Baldo, B. A., & Kelley, A. E. (2007). Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology, 191(3), 439–459. Balleine, B. W., & O’Doherty, J. P. (2010). Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology, 35(1), 48–69. Barnard, M. (2005). Drugs in the family: The impact on parents and siblings. London: Joseph Rowntree Foundation. Barrós-Loscertales, A., Bustamante, J.-C. C., Ventura-Campos, N., Llopis, J.-J. J., Parcet, M.-A. A., Avila, C., & Barros-Loscertales, A. (2011). Lower activation in the right frontoparietal network during a counting Stroop task in a cocaine-dependent group. Psychiatry Research, 194(2), 111–118. Baxter, L. R., Schwartz, J. M., Mazziotta, J. C., Phelps, M. E., Pahl, J. J., Guze, B. H., & Fairbanks, L. (1988). Cerebral glucose metabolic rates in nondepressed patients with obsessive-compulsive disorder. American Journal of Psychiatry, 145(12), 1560– 1563. Bechara, A. (2005). Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience, 8(11), 1458–1463. Bechara, A., Damasio, A. R., Damasio, H., & Anderson, S. W. (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50(13), 7–15. Bechara, A., & Damasio, H. (2002). Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia, 40(10), 1675–1689. Bechara, A., Damasio, H., & Damasio, A. R. (2000). Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex, 10(3), 295–307. Bechara, A., Dolan, S., Denburg, N., Hindes, A., Anderson, S. W., & Nathan, P. E. (2001). Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia, 39(4), 376– 389. Beck, A., Schlagenhauf, F., Wüstenberg, T., Hein, J., Kienast, T., Kahnt, T., … Wrase, J. (2009). Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry, 66(8), 734–742. 158 References Beck, A., Steer, R. A., Ball, R., & Ranieri, W. (1996). Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment, 67(3), 588–597. Beck, A. T., Steer, R. A., & Garbin, M. G. (1988). Psychometric Properties of the Beck Depression Inventory - 25 Years of Evaluation. Clinical Psychology Review, 8(1), 77–100. Belin, D., & Everitt, B. J. (2008). Cocaine seeking habits depend upon dopaminedependent serial connectivity linking the ventral with the dorsal striatum. Neuron, 57(3), 432–441. Belin, D., Mar, A. C., Dalley, J. W., Robbins, T. W., & Everitt, B. J. (2008). High impulsivity predicts the switch to compulsive cocaine-taking. Science, 320(5881), 1352–1355. Berridge, K. C., & Robinson, T. E. (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews, 28(3), 309–369. Beveridge, T. J. R., Smith, H. R., Daunais, J. B., Nader, M. A., & Porrino, L. J. (2006). Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. European Journal of Neuroscience, 23(11), 3109–3118. Bjork, J. M., Smith, A. R., & Hommer, D. W. (2008). Striatal sensitivity to reward deliveries and omissions in substance dependent patients. NeuroImage, 42(4), 1609– 1621. Blum, K., Braverman, E. R., Holder, J. M., Lubar, J. F., Monastra, V. J., Miller, D., … Comings, D. E. (2000). Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. Journal of Psychoactive Drugs, 32, Supplement. Blum, K., Cull, J. G., Braverman, E. R., & Comings, D. E. (1996). Reward deficiency syndrome. American Scientist, 84(2), 132–145. Blum, K., Noble, E. P., Sheridan, P. J., Montgomery, A., Ritchie, T., Jagadeeswaran, P., … Cohn, J. B. (1990). Allelic association of human dopamine D2 receptor gene in alcoholism. Journal of the American Medical Association, 263(15), 2055–2060. Bolla, K. I., Eldreth, D. A., London, E. D., Kiehl, K. A., Mouratidis, M., Contoreggi, C., … Ernst, M. (2003). Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage, 19(3), 1085–1094. Bolla, K. I., Ernst, M., Kiehl, K., Mouratidis, M., Eldreth, D., Contoreggi, C., … London, E. (2004). Prefrontal cortical dysfunction in abstinent cocaine abusers. Journal of Neuropsychiatry and Clinical Neuroscience, 16(4), 456–464. 159 References Breiter, H. C., Gollub, R. L., Weisskoff, R. M., Kennedy, D. N., Makris, N., Berke, J. D., … Hyman, S. E. (1997). Acute effects of cocaine on human brain activity and emotion. Neuron, 19(3), 591–611. Brewer, J. A., Worhunsky, P. D., Carroll, K. M., Rounsaville, B. J., & Potenza, M. N. (2008). Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biological Psychiatry, 64(11), 998–1004. Bullmore, E. T., Brammer, M. J., Rabe-Hesketh, S., Curtis, V. A., Morris, R. G., Williams, S. C., … McGuire, P. K. (1999). Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Human Brain Mapping, 7(1), 38–48. Bullmore, E. T., Long, C., Suckling, J., Fadili, J., Calvert, G., Zelaya, F., … Brammer, M. (2001). Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Human Brain Mapping, 12(2), 61–78. Bunge, S. A., Ochsner, K. N., Desmond, J. E., Glover, G. H., & Gabrieli, J. D. E. (2001). Prefrontal regions involved in keeping information in and out of mind. Brain, 124, 2074–2086. Burns, G. L., Keortge, S. G., Formea, G. M., & Sternberger, L. G. (1996). Revision of the Padua Inventory of obsessive compulsive disorder symptoms: Distinctions between worry, obsessions, and compulsions. Behaviour Research and Therapy. Camarena, B., Santiago, H., Aguilar, A., Ruvinskis, E., González-Barranco, J., & Nicolini, H. (2004). Family-based association study between the monoamine oxidase A gene and obesity: implications for psychopharmacogenetic studies. Neuropsychobiology, 49(3), 126–129. Cambridge, V. C., Ziauddeen, H., Nathan, P. J., Subramaniam, N., Dodds, C., Chamberlain, S. R., … Fletcher, P. C. (2013). Neural and behavioral effects of a novel mu opioid receptor antagonist in binge-eating obese people. Biological Psychiatry, 73(9), 887–894. Carpenter, K. M., Schreiber, E., Church, S., & McDowell, D. (2006). Drug Stroop performance: relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addictive Behaviors, 31(1), 174–181. Carver, C. S., & White, T. L. (1994). Behavioral-inhibition, behavioral activation, and affective responses to impending reward and punishment - the Bis Bas scales. Journal of Personality and Social Psychology, 67(2), 319–333. Caspi, A., McClay, J., Moffitt, T. E., Mill, J., Martin, J., Craig, I. W., … Poulton, R. (2002). Role of genotype in the cycle of violence in maltreated children. Science, 297(5582), 851–854. Castellanos, F. X., Giedd, J. N., Marsh, W. L., Hamburger, S. D., Vaituzis, A. C., Dickstein, D. P., … Rapoport, J. L. (1996). Quantitative brain magnetic resonance 160 References imaging in attention-deficit hyperactivity disorder. Archives of General Psychiatry, 53(7), 607–616. Cavazos-Rehg, P. A., Spitznagel, E. L., Schootman, M., Strickland, J. R., Afful, S. E., Cottler, L. B., & Bierut, L. J. (2009). Risky sexual behaviors and sexually transmitted diseases: a comparison study of cocaine-dependent individuals in treatment versus a community-matched sample. AIDS Patient Care and STDs, 23(9), 727–734. Chang, L., Cloak, C., Patterson, K., Grob, C., Miller, E. N., & Ernst, T. (2005). Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biological Psychiatry, 57(9), 967–974. Childress, A. R., Mozley, P. D., McElgin, W., Fitzgerald, J., Reivich, M., & O’Brien, C. P. (1999). Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry, 156(1), 11–18. Clark, L., Bechara, A., Damasio, H., Aitken, M. R. F., Sahakian, B. J., & Robbins, T. W. (2008). Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain, 131(Pt 5), 1311–1322. Clark, L., Robbins, T. W., Ersche, K. D., & Sahakian, B. J. (2006). Reflection impulsivity in current and former substance users. Biological Psychiatry, 60(5), 515–522. Colzato, L. S., Huizinga, M., & Hommel, B. (2009). Recreational cocaine polydrug use impairs cognitive flexibility but not working memory. Psychopharmacology, 207(2), 225–234. Colzato, L. S., van den Wildenberg, W. P. M., & Hommel, B. (2007). Impaired inhibitory control in recreational cocaine users. Plos One, 2(11), e1143. Comings, D. E., Flanagan, S. D., Dietz, G., Muhleman, D., Knell, E., & Gysin, R. (1993). The dopamine-D(2) receptor (Drd2) as a major gene in obesity and height. Biochemical Medicine and Metabolic Biology, 50(2), 176–185. Comings, D. E., Muhleman, D., Ahn, C., Gysin, R., & Flanagan, S. D. (1994). The dopamine-D(2) receptor gene - a genetic risk factor in substance-abuse. Drug and Alcohol Dependence, 34(3), 175–180. Cools, R., Clark, L., Owen, A. M., & Robbins, T. W. (2002). Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. Journal of Neuroscience, 22(11), 4563–4567. Cools, R., Sheridan, M., Jacobs, E., & D’Esposito, M. (2007). Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. Journal of Neuroscience, 27(20), 5506–5514. Copersino, M. L., Serper, M. R., Vadhan, N., Goldberg, B. R., Richarme, D., Chou, J. C., … Cancro, R. (2004). Cocaine craving and attentional bias in cocaine-dependent schizophrenic patients. Psychiatry Research, 128(3), 209–218. 161 References Cox, W. M., Fadardi, J. S., & Pothos, E. M. (2006). The addiction-stroop test: theoretical considerations and procedural recommendations. Psychological Bulletin, 132(3), 443–476. Daglish, M. R. C., & Nutt, D. J. (2003). Brain imaging studies in human addicts. European Neuropsychopharmacology, 13(6), 453–458. Daglish, M. R. C., Weinstein, A., Malizia, A. L., Wilson, S., Melichar, J. K., Britten, S., … Nutt, D. J. (2001). Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. American Journal of Psychiatry, 158(10), 1680–1686. Dalley, J. W., Everitt, B. J., & Robbins, T. W. (2011). Impulsivity, compulsivity, and topdown cognitive control. Neuron, 69(4), 680–694. Dalley, J. W., Fryer, T. D., Brichard, L., Robinson, E. S. J., Theobald, D. E. H., Lääne, K., … Robbins, T. W. (2007). Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science, 315(5816), 1267–1270. Davis, C., & Carter, J. C. (2009). Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite, 53(1), 1–8. Davis, C., & Fox, J. (2008). Sensitivity to reward and body mass index (BMI): evidence for a non-linear relationship. Appetite, 50(1), 43–49. Davis, C., Levitan, R. D., Muglia, P., Bewell, C., & Kennedy, J. L. (2004). Decisionmaking deficits and overeating: a risk model for obesity. Obesity Research, 12(6), 929–935. Davis, C., Levitan, R. D., Reid, C., Carter, J. C., Kaplan, A. S., Patte, K. A., … Kennedy, J. L. (2009). Dopamine for “wanting” and opioids for “liking”: a comparison of obese adults with and without binge eating. Obesity, 17(6), 1220–1225. Davis, C., Strachan, S., & Berkson, M. (2004). Sensitivity to reward: implications for overeating and overweight. Appetite, 42(2), 131–138. Deadwyler, S. A. (2010). Electrophysiological correlates of abused drugs: relation to natural rewards. Annals of the New York Academy of Sciences, 1187(1), 140–147. Di Chiara, G. (1999). Drug addiction as dopamine-dependent associative learning disorder. European Journal of Pharmacology, 375(1-3), 13–30. Driscoll, I., Beydoun, M. A., An, Y., Davatzikos, C., Ferrucci, L., Zonderman, A. B., & Resnick, S. M. (2012). Midlife obesity and trajectories of brain volume changes in older adults. Human Brain Mapping, 33(9), 2204–2210. Ducci, F., Newman, T. K., Funt, S., Brown, G. L., Virkkunen, M., & Goldman, D. (2006). A functional polymorphism in the MAOA gene promoter (MAOA-LPR) 162 References predicts central dopamine function and body mass index. Molecular Psychiatry, 11(9), 858–866. Ehrman, R. N., Robbins, S. J., Childress, A. R., & O’Brien, C. P. (1992). Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology, 107(4), 523–529. Elias, M. F., Elias, P. K., Sullivan, L. M., Wolf, P. A., & D’Agostino, R. B. (2003). Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. International Journal of Obesity, 27(2), 260–268. Elliott, R., Dolan, R. J., & Frith, C. D. (2000). Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cerebral Cortex, 10(3), 308–317. Ersche, K. D., Barnes, A., Jones, P. S., Morein-Zamir, S., Robbins, T. W., & Bullmore, E. T. (2011). Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain, 134, 2013– 2024. Ersche, K. D., Bullmore, E. T., Craig, K. J., Shabbir, S. S., Abbott, S., Müller, U., … Robbins, T. W. (2010). Influence of compulsivity of drug abuse on dopaminergic modulation of attentional bias in stimulant dependence. Archives of General Psychiatry, 67(6), 632–644. Ersche, K. D., Clark, L., London, M., Robbins, T. W., & Sahakian, B. J. (2006). Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology, 31(5), 1036–1047. Ersche, K. D., Fletcher, P. C., Lewis, S. J. G., Clark, L., Stocks-Gee, G., London, M., … Sahakian, B. J. (2005). Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology, 180(4), 612–623. Ersche, K. D., Fletcher, P. C., Roiser, J. P., Fryer, T. D., London, M., Robbins, T. W., & Sahakian, B. J. (2006). Differences in orbitofrontal activation during decisionmaking between methadone-maintained opiate users, heroin users and healthy volunteers. Psychopharmacology, 188(3), 364–73. Ersche, K. D., Jones, P. S., Williams, G. B., Robbins, T. W., & Bullmore, E. T. (2013). Cocaine dependence: a fast-track for brain ageing? Molecular Psychiatry, 18(2), 134–135. Ersche, K. D., Jones, P. S., Williams, G. B., Smith, D. G., Bullmore, E. T., & Robbins, T. W. (2013). Distinctive personality traits and neural correlates associated with stimulant drug use versus familial risk of stimulant dependence. Biological Psychiatry, 74(2), 137–144. 163 References Ersche, K. D., Jones, P. S., Williams, G. B., Turton, A. J., Robbins, T. W., & Bullmore, E. T. (2012). Abnormal brain structure implicated in stimulant drug addiction. Science, 335(6068), 601–604. Ersche, K. D., & Robbins, T. W. (2011). An integrated framework for human neuroimaging studies of addiction from a preclinical perspective. In B. Adinoff & E. A. Stein (Eds.), Neuroimaging in Addiction (pp. 7–35). Chichester, UK: John Wiley & Sons, Ltd. Ersche, K. D., Roiser, J. P., Robbins, T. W., & Sahakian, B. J. (2008). Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology, 197(3), 421–431. Ersche, K. D., Turton, A. J., Chamberlain, S. R., Muller, U., Bullmore, E. T., & Robbins, T. W. (2012). Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. American Journal of Psychiatry, 169(9), 926– 936. Ersche, K. D., Turton, A. J., Pradhan, S., Bullmore, E. T., & Robbins, T. W. (2010). Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biological Psychiatry, 68(8), 770–773. Ersche, K. D., Williams, G. B., Robbins, T. W., & Bullmore, E. T. (2013). Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Current Opinion in Neurobiology, 23(4), 615–624. Everitt, B. J. (1990). Sexual motivation: A neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neuroscience and Biobehavioral Reviews, 14(2), 217–232. Everitt, B. J., Belin, D., Economidou, D., Pelloux, Y., Dalley, J. W., & Robbins, T. W. (2008). Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society, Series B Biological Sciences, 363(1507), 3125–3135. Everitt, B. J., Hutcheson, D. M., Ersche, K. D., Pelloux, Y., Dalley, J. W., & Robbins, T. W. (2007). The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Annals of the New York Academy of Sciences, 1121, 576–597. Everitt, B. J., & Robbins, T. W. (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience, 8(11), 1481– 1489. Fairburn, C. G., & Beglin, S. J. (1994). Assessment of eating disorders: interview or selfreport questionnaire? The International Journal of Eating Disorders, 16(4), 363– 370. 164 References Felsted, J. A., Ren, X., Chouinard-Decorte, F., & Small, D. M. (2010). Genetically determined differences in brain response to a primary food reward. Journal of Neuroscience, 30(7), 2428–2432. Field, M. (2005). Cannabis “dependence” and attentional bias for cannabis-related words. Behavioural Pharmacology, 16(5-6), 473–476. Field, M., Christiansen, P., Cole, J., & Goudie, A. (2007). Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction, 102(4), 579–586. Field, M., & Cox, W. M. (2008). Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug and Alcohol Dependence, 97(1-2), 1– 20. First, M. B., Spitzer, R. L., Gibbon, M., & Williams, J. B. W. (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders (Research V.). New York: New York State Psychiatric Institute, Biometrics Research. FitzGerald, T. H. B., Seymour, B., & Dolan, R. J. (2009). The role of human orbitofrontal cortex in value comparison for incommensurable objects. Journal of Neuroscience, 29(26), 8388–8395. Fox, H. C., Axelrod, S. R., Paliwal, P., Sleeper, J., & Sinha, R. (2007). Difficulties in emotion regulation and impulse control during cocaine abstinence. Drug and Alcohol Dependence, 89(2-3), 298–301. Franken, I. (2003). Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 27(4), 563–579. Franken, I. H. A., Hendriksa, V. M., & van den Brink, W. (2002). Initial validation of two opiate craving questionnaires the obsessive compulsive drug use scale and the desires for drug questionnaire. Addictive Behaviors, 27(5), 675–685. Franken, I. H. A., Kroon, L. Y., & Hendriks, V. M. (2000). Influence of individual differences in craving and obsessive cocaine thoughts on attentional processes in cocaine abuse patients. Addictive Behaviors, 25(1), 99–102. Franklin, T. R., Acton, P. D., Maldjian, J. A., Gray, J. D., Croft, J. R., Dackis, C. A., … Childress, A. R. (2002). Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biological Psychiatry, 51(2), 134–142. Garavan, H., Kaufman, J. N., & Hester, R. (2008). Acute effects of cocaine on the neurobiology of cognitive control. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1507), 3267–3276. Garavan, H., Pankiewicz, J., Bloom, A., Cho, J. K., Sperry, L., Ross, T. J., … Stein, E. A. (2000). Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry, 157(11), 1789–1798. 165 References Gerra, G., Baldaro, B., Zaimovic, A., Moi, G., Bussandri, M., Raggi, M. A., & Brambilla, F. (2003). Neuroendocrine responses to experimentally-induced emotions among abstinent opioid-dependent subjects. Drug and Alcohol Dependence, 71(1), 25–35. Giuliano, C., Robbins, T. W., Nathan, P. J., Bullmore, E. T., & Everitt, B. J. (2012). Inhibition of opioid transmission at the μ-opioid receptor prevents both food seeking and binge-like eating. Neuropsychopharmacology, 37(12), 2643–2652. Glover, G. H. (1999). Deconvolution of impulse response in event-related BOLD fMRI. NeuroImage, 9(4), 416–429. Goldstein, R. Z., Alia-Klein, N., Tomasi, D., Carrillo, J. H., Maloney, T., Woicik, P. A., … Volkow, N. D. (2009). Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proceedings of the National Academy of Sciences of the United States of America, 106(23), 9453–9458. Goldstein, R. Z., Alia-Klein, N., Tomasi, D., Zhang, L., Cottone, L. A., Maloney, T., … Volkow, N. D. (2007). Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? American Journal of Psychiatry, 164(1), 43–51. Goldstein, R. Z., Tomasi, D., Alia-Klein, N., Cottone, L. A., Zhang, L., Telang, F., & Volkow, N. D. (2007). Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug and Alcohol Dependence, 87(2-3), 233–240. Goldstein, R. Z., Tomasi, D., Rajaram, S., Cottone, L. A., Zhang, L., Maloney, T., … Volkow, N. D. (2007). Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience, 144(4), 1153–1159. Goldstein, R. Z., & Volkow, N. D. (2002). Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry, 159(10), 1642–1652. Goldstein, R. Z., & Volkow, N. D. (2011). Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Reviews: Neuroscience, 12(11), 652–669. Goldstein, R. Z., Volkow, N. D., Wang, G. J., Fowler, J. S., & Rajaram, S. (2001). Addiction changes orbitofrontal gyrus function: Involvement in response inhibition. NeuroReport, 12(11), 2595–2599. Gooding, D. C., Burroughs, S., & Boutros, N. N. (2008). Attentional deficits in cocainedependent patients: converging behavioral and electrophysiological evidence. Psychiatry Research, 160(2), 145–154. Goodman, W. K., Price, L. H., Rasmussen, S. A., Mazure, C., Delgado, P., Heninger, G. R., & Charney, D. S. (1989). The Yale-Brown Obsessive Compulsive Scale. II. Validity. Archives of General Psychiatry, 46(11), 1012–1016. 166 References Gordon, L. (2006). The economic and social costs of Class A drug use in England and Wales. London. Gormally, J., Black, S., Daston, S., & Rardin, D. (1982). The assessment of binge eating severity among obese persons. Addictive Behaviors, 7(1), 47–55. Gottesman, I. I., & Gould, T. D. (2003). The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry, 160(4), 636– 645. Gottfried, J. A., O’Doherty, J., & Dolan, R. J. (2003). Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science, 301(5636), 1104–1107. Grant, S., Contoreggi, C., & London, E. D. (2000). Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia, 38(8), 1180–1187. Grant, S., London, E. D., Newlin, D. B., Villemagne, V. L., Liu, X., Contoreggi, C., … Margolin, A. (1996). Activation of memory circuits during cue-elicited cocaine craving. Proceedings of the National Academy of Sciences of the United States of America, 93(21), 12040–12045. Guindalini, C., Howard, M., Haddley, K., Laranjeira, R., Collier, D., Ammar, N., … Breen, G. (2006). A dopamine transporter gene functional variant associated with cocaine abuse in a Brazilian sample. Proceedings of the National Academy of Sciences of the United States of America, 103(12), 4552–4557. Gunstad, J., Lhotsky, A., Wendell, C. R., Ferrucci, L., & Zonderman, A. B. (2010). Longitudinal examination of obesity and cognitive function: results from the Baltimore longitudinal study of aging. Neuroepidemiology, 34(4), 222–229. Gunstad, J., Paul, R. H., Cohen, R. A., Tate, D. F., Spitznagel, M. B., & Gordon, E. (2007). Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Comprehensive Psychiatry, 48(1), 57–61. Gunstad, J., Paul, R. H., Cohen, R. A., Tate, D. F., Spitznagel, M. B., Grieve, S., & Gordon, E. (2008). Relationship between body mass index and brain volume in healthy adults. The International Journal of Neuroscience, 118(11), 1582–1593. Hall, F. S., Drgonova, J., Jain, S., & Uhl, G. R. (2013). Implications of genome wide association studies for addiction: Are our a priori assumptions all wrong? Pharmacology & Therapeutics, 140(3), 267–279. Hammers, A., Allom, R., Koepp, M. J., Free, S. L., Myers, R., Lemieux, L., … Duncan, J. S. (2003). Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Human Brain Mapping, 19(4), 224–247. Hanlon, C. A., Dufault, D. L., Wesley, M. J., & Porrino, L. J. (2011). Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology, 218(4), 681–692. 167 References Hernandez, L., & Hoebel, B. G. (1988). Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sciences, 42(18), 1705–1712. Hester, R., Dixon, V., & Garavan, H. (2006). A consistent attentional bias for drugrelated material in active cocaine users across word and picture versions of the emotional Stroop task. Drug and Alcohol Dependence, 81(3), 251–257. Hester, R., & Garavan, H. (2004). Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. Journal of Neuroscience, 24(49), 11017–11022. Ho, A. J., Raji, C. A., Becker, J. T., Lopez, O. L., Kuller, L. H., Hua, X., … Thompson, P. M. (2010). Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiology of Aging, 31(8), 1326–1339. Horner, M. D. (1999). Attentional functioning in abstinent cocaine abusers. Drug and Alcohol Dependence, 54(1), 19–33. Horstmann, A., Busse, F. P., Mathar, D., Müller, K., Lepsien, J., Schlögl, H., … Pleger, B. (2011). Obesity-related differences between women and men in brain structure and goal-directed behavior. Frontiers in Human Neuroscience, 5, 58. Hwang, L. Y., Ross, M. W., Zack, C., Bull, L., Rickman, K., & Holleman, M. (2000). Prevalence of sexually transmitted infections and associated risk factors among populations of drug abusers. Clinical Infectious Diseases, 31(4), 920–926. Ifland, J. R., Preuss, H. G., Marcus, M. T., Rourke, K. M., Taylor, W. C., Burau, K., … Manso, G. (2009). Refined food addiction: a classic substance use disorder. Medical Hypotheses, 72(5), 518–526. Ito, R., Dalley, J. W., Howes, S. R., Robbins, T. W., & Everitt, B. J. (2000). Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. Journal of Neuroscience, 20(19), 7489–7495. Ito, R., Dalley, J. W., Robbins, T. W., & Everitt, B. J. (2002). Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drugassociated cue. Journal of Neuroscience, 22(14), 6247–6253. Izquierdo, A., Suda, R. K., & Murray, E. A. (2004). Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. Journal of Neuroscience, 24(34), 7540–7548. Jacobsen, L. K., Giedd, J. N., Gottschalk, C., Kosten, T. R., & Krystal, J. H. (2001). Quantitative morphology of the caudate and putamen in patients with cocaine dependence. American Journal of Psychiatry, 158(3), 486–489. 168 References Jenkinson, M., Bannister, P., Brady, M., & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. Jenkinson, M., & Smith, S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–156. Jentsch, D., Olausson, P., De La Garza, R., & Taylor, J. R. (2002). Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology, 26(2), 183–190. Jentsch, D., & Taylor, J. R. (1999). Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology, 146(4), 373–390. Jia, Z., Worhunsky, P. D., Carroll, K. M., Rounsaville, B. J., Stevens, M. C., Pearlson, G. D., & Potenza, M. N. (2011). An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biological Psychiatry, 70(6), 553–560. Joel, D., Doljansky, J., Roz, N., & Rehavi, M. (2005). Role of the orbital cortex and of the serotonergic system in a rat model of obsessive compulsive disorder. Neuroscience, 130(1), 25–36. Jonsson, E. G., Nothen, M. M., Grunhage, F., Farde, L., Nakashima, Y., Propping, P., & Sedvall, G. C. (1999). Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Molecular Psychiatry, 4(3), 290–296. Kaufman, J. N., Ross, T. J., Stein, E. A., & Garavan, H. (2003). Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. Journal of Neuroscience, 23(21), 7839–7843. Kerns, J. G., Cohen, J. D., MacDonald 3rd, A. W., Cho, R. Y., Stenger, V. A., & Carter, C. S. (2004). Anterior cingulate conflict monitoring and adjustments in control. Science, 303(5660), 1023–1026. Kim, H., Shimojo, S., & O’Doherty, J. P. (2011). Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cerebral Cortex, 21(4), 769–776. Kirby, K. N., & Petry, N. M. (2004). Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction, 99, 461–471. Kirby, K. N., Petry, N. M., & Bickel, W. K. (1999). Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology, 128(1), 78–87. 169 References Knutson, B., Adams, C. M., Fong, G. W., & Hommer, D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, 21(16), RC159. Knutson, B., Fong, G. W., Adams, C. M., Varner, J. L., & Hommer, D. (2001). Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport, 12(17), 3683–3687. Knutson, B., Westdorp, A., Kaiser, E., & Hommer, D. (2000). FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage, 12(1), 20–27. Koller, G., Bondy, B., Preuss, U. W., Bottlender, M., & Soyka, M. (2003). No association between a polymorphism in the promoter region of the MAOA gene with antisocial personality traits in alcoholics. Alcohol and Alcoholism, 38(1), 31–34. Koob, G. F., Ahmed, S. H., Boutrel, B., Chen, S. A., Kenny, P. J., Markou, A., … Sanna, P. P. (2004). Neurobiological mechanisms in the transition from drug use to drug dependence. Neuroscience and Biobehavioral Reviews, 27(8), 739–749. Koob, G. F., & Le Moal, M. (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology, 24(2), 97–129. Koob, G. F., & Le Moal, M. (2005). Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nature Neuroscience, 8(11), 1442–1444. Koob, G. F., & Nestler, E. J. (1997). The neurobiology of drug addiction. Journal of Neuropsychiatry and Clinical Neurosciences, 9(3), 482–497. Koob, G. F., & Volkow, N. D. (2010). Neuropsychopharmacology, 35(1), 217–238. Neurocircuitry of addiction. Lee, B., London, E. D., Poldrack, R. A., Farahi, J., Nacca, A., Monterosso, J. R., … Mandelkern, M. A. (2009). Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. Journal of Neuroscience, 29(47), 14734–40. Levine, A. J., Hardy, D. J., Miller, E., Castellon, S. A., Longshore, D., & Hinkin, C. H. (2006). The effect of recent stimulant use on sustained attention in HIV-infected adults. Journal of Clinical and Experimental Neuropsychology, 28(1), 29–42. Levy, D. J., & Glimcher, P. W. (2012). The root of all value: a neural common currency for choice. Current Opinion in Neurobiology, 22(6), 1027–1038. Lim, K. O., Choi, S. J., Pomara, N., Wolkin, A., & Rotrosen, J. P. (2002). Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biological Psychiatry, 51(11), 890–895. 170 References Liu, X., Matochik, J. A., Cadet, J. L., & London, E. D. (1998). Smaller volume of prefrontal lobe in polysubstance abusers: A magnetic resonance imaging study. Neuropsychopharmacology, 18(4), 243–252. Logan, G. D., Schachar, R. J., & Tannock, R. (1997). Impulsivity and inhibitory control. Psychological Science, 8(1), 60–64. London, E. D., Berman, S. M., Voytek, B., Simon, S. L., Mandelkern, M. A., Monterosso, J., … Ling, W. (2005). Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biological Psychiatry, 58(10), 770– 778. London, E. D., Ernst, M., Grant, S., Bonson, K., & Weinstein, A. (2000). Orbitofrontal cortex and human drug abuse: functional imaging. Cerebral Cortex, 10(3), 334–342. Maayan, L., Hoogendoorn, C., Sweat, V., & Convit, A. (2011). Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity, 19(7), 1382–1387. MacDonald, A. W., Cohen, J. D., Stenger, V. A., & Carter, C. S. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288(5472), 1835–1838. Marissen, M. A. E., Franken, I. H. A., Waters, A. J., Blanken, P., van den Brink, W., & Hendriks, V. M. (2006). Attentional bias predicts heroin relapse following treatment. Addiction, 101(9), 1306–1312. Martinez, D., Broft, A., Foltin, R. W., Slifstein, M., Hwang, D. R., Huang, Y., … Laruelle, M. (2004). Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology, 29(6), 1190–1202. Martinez, D., Greene, K., Broft, A., Kumar, D., Liu, F., Narendran, R., … Kleber, H. D. (2009). Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. American Journal of Psychiatry, 166(10), 1170–1177. Matochik, J. A., London, E. D., Eldreth, D. A., Cadet, J. L., & Bolla, K. I. (2003). Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. NeuroImage, 19(3), 1095–1102. Menzies, L., Chamberlain, S. R., Laird, A. R., Thelen, S. M., Sahakian, B. J., & Bullmore, E. T. (2008). Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofrontostriatal model revisited. Neuroscience and Biobehavioral Reviews, 32(3), 525–549. Meunier, D., Ersche, K. D., Craig, K. J., Fornito, A., Merlo-Pich, E., Fineberg, N. A., … Bullmore, E. T. (2011). Brain functional connectivity in stimulant drug dependence and obsessive-compulsive disorder. NeuroImage. 171 References Meyer-Lindenberg, A., Buckholtz, J. W., Kolachana, B., R Hariri, A., Pezawas, L., Blasi, G., … Weinberger, D. R. (2006). Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Sciences of the United States of America, 103(16), 6269–6274. Miller, G. A., & Chapman, J. P. (2001). Misunderstanding analysis of covariance. Journal of Abnormal Psychology, 110(1), 40–48. Monterosso, J. R., Aron, A. R., Cordova, X., Xu, J., & London, E. D. (2005). Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and Alcohol Dependence, 79(2), 273–277. Nader, M. A., Czoty, P. W., Gould, R. W., & Riddick, N. V. (2008). Positron emission tomography imaging studies of dopamine receptors in primate models of addiction. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 363(1507), 3223–3232. National Treatment Agency for Substance Misuse. (2012). Drug Treatment 2012: Progress Made, Challenges Ahead. London. Nelson, H. E. (1982). The National Adult Reading Test Manual. Windsor: NFER-Nelson. Nestor, L., Hester, R., & Garavan, H. (2010). Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. NeuroImage, 49(1), 1133– 1143. Nestor, L. J., Ghahremani, D. G., Monterosso, J., & London, E. D. (2011). Prefrontal hypoactivation during cognitive control in early abstinent methamphetaminedependent subjects. Psychiatry Research: Neuroimaging, 194(3), 287–295. Nigg, J. T., Wong, M. M., Martel, M. M., Jester, J. M., Puttler, L. I., Glass, J. M., … Zucker, R. A. (2006). Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 45(4), 468–475. Noble, E. P. (2000). Addiction and its reward process through polymorphisms of the D2 dopamine receptor gene: a review. European Psychiatry, 15(2), 79–89. Noble, E. P., Blum, K., Khalsa, M. E., Ritchie, T., Montgomery, A., Wood, R. C., … Anglin, M. D. (1993). Allelic association of the D2 dopamine receptor gene with cocaine dependence. Drug and Alcohol Dependence, 33(3), 271–285. Noble, E. P., Gottschalk, L. A., Fallon, J. H., Ritchie, T. L., & Wu, J. C. (1997). D2 dopamine receptor polymorphism and brain regional glucose metabolism. American Journal of Medical Genetics, 74(2), 162–166. Noonan, M. P., Mars, R. B., & Rushworth, M. F. S. (2011). Distinct roles of three frontal cortical areas in reward-guided behavior. Journal of Neuroscience, 31(40), 14399– 14412. 172 References Nutt, D. J. (1996). Addiction: brain mechanisms and their treatment implications. The Lancet, 347(8993), 31–36. Nutt, D. J. (2013). Addiction: lifestyle choice or medical diagnosis? Journal of Evaluation in Clinical Practice, 19(3), 6269–6274. O’Doherty, J. (2002). Role of human orbitofrontal cortex in representing stimulus-reward value. Psychophysiology, 39, S4–S4. Opris, I., Hampson, R. E., & Deadwyler, S. A. (2009). The encoding of cocaine vs. natural rewards in the striatum of nonhuman primates: categories with different activations. Neuroscience, 163(1), 40–54. Pannacciulli, N., Del Parigi, A., Chen, K., Le, D. S., Reiman, E. M., & Tataranni, P. A. (2006). Brain abnormalities in human obesity: a voxel-based morphometric study. NeuroImage, 31(4), 1419–1425. Parylak, S. L., Koob, G. F., & Zorrilla, E. P. (2011). The dark side of food addiction. Physiology and Behavior, 104(1), 149–156. Patton, J. H., Stanford, M. S., & Barratt, E. S. (1995). Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology, 51(6), 768–774. Pelchat, M. L. (2002). Of human bondage: food craving, obsession, compulsion, and addiction. Physiology and Behavior, 76(3), 347–352. Perry, J. L., & Carrol, M. E. (2008). The role of impulsive behavior in drug abuse. Psychopharmacology, 200(1), 1–26. Philibert, R. A., Gunter, T. D., Beach, S. R. H., Brody, G. H., & Madan, A. (2008). MAOA methylation is associated with nicotine and alcohol dependence in women. American Journal of Medical Genetics, 147B(5), 565–570. Porrino, L. J., Daunais, J. B., Smith, H. R., & Nader, M. A. (2004). The expanding effects of cocaine: studies in a nonhuman primate model of cocaine self-administration. Neuroscience and Biobehavioral Review, 27(8), 813–820. Porrino, L. J., Lyons, D., Miller, M. D., Smith, H. R., Friedman, D. P., Daunais, J. B., & Nader, M. A. (2002). Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. Journal of Neuroscience, 22(17), 7687–7694. Porrino, L. J., Smith, H. R., Nader, M. A., & Beveridge, T. J. R. (2007). The effects of cocaine: a shifting target over the course of addiction. Progress in NeuroPsychopharmacology & Biological Psychiatry, 31(8), 1593–600. Rada, P., Avena, N. M., & Hoebel, B. G. (2005). Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience, 134(3), 737–744. 173 References Raji, C. A., Ho, A. J., Parikshak, N. N., Becker, J. T., Lopez, O. L., Kuller, L. H., … Thompson, P. M. (2010). Brain structure and obesity. Human Brain Mapping, 31(3), 353–364. Reynolds, B., Ortengren, A., Richards, J. B., & de Wit, H. (2006). Dimensions of impulsive behavior: personality and behavioral measures. Personality and Individual Differences, 40(2), 305–315. Robbins, T. W., Ersche, K. D., & Everitt, B. J. (2008). Drug addiction and the memory systems of the brain. Annals of the New York Academy of Sciences, 1141, 1–21. Robbins, T. W., Gillan, C. M., Smith, D. G., de Wit, S., & Ersche, K. D. (2012). Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends in Cognitive Sciences, 16(1), 81–91. Robinson, T. E., & Berridge, K. C. (1993). The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews, 18, 247–291. Rogers, R. D., & Robbins, T. W. (2001). Investigating the neurocognitive deficits associated with chronic drug misuse. Current Opinion in Neurobiology, 11(2), 250– 257. Rolls, E. T. (2000). The orbitofrontal cortex and reward. Cereb Cortex, 10(3), 284–294. Rushworth, M. F. S., & Behrens, T. E. J. (2008). Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience, 11(4), 389–397. Rushworth, M. F. S., Behrens, T. E. J., Rudebeck, P. H., & Walton, M. E. (2007). Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends in Cognitive Sciences, 11(4), 168–176. Rushworth, M. F. S., Noonan, M. P., Boorman, E. D., Walton, M. E., & Behrens, T. E. (2011). Frontal cortex and reward-guided learning and decision-making. Neuron, 70(6), 1054–1069. Saito, T., Lachman, H. M., Diaz, L., Hallikainen, T., Kauhanen, J., Salonen, J. T., … Tiihonen, J. (2002). Analysis of monoamine oxidase A (MAOA) promoter polymorphism in Finnish male alcoholics. Psychiatry Research, 109(2), 113–119. Salo, R., Fassbender, C., Buonocore, M. H., & Ursu, S. (2013). Behavioral regulation in methamphetamine abusers: An fMRI study. Psychiatry Research: Neuroimaging, 211(3), 234–238. Salo, R., Nordahl, T. E., Possin, K., Leamon, M., Gibson, D. R., Galloway, G. P., … Sullivan, E. V. (2002). Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Research, 111(1), 65–74. Saunders, J. B., Aasland, O. G., Babor, T. F., Delafuente, J. R., & Grant, M. (1993). Development of the Alcohol-Use Disorders Identification Test (AUDIT) - WHO 174 References collaborative project on early detection of persons with harmful alcoholconsumption-II. Addiction, 88(6), 791–804. Schoenbaum, G., Roesch, M. R., & Stalnaker, T. A. (2006). Orbitofrontal cortex, decision-making and drug addiction. Trends in Neurosciences, 29(2), 116–124. Schoenbaum, G., & Setlow, B. (2005). Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cerebral Cortex, 15(8), 1162–1169. Schoenbaum, G., & Shaham, Y. (2008). The role of orbitofrontal cortex in drug addiction: A review of preclinical studies. Biological Psychiatry, 63(3), 256–262. Schott, B. H., Minuzzi, L., Krebs, R. M., Elmenhorst, D., Lang, M., Winz, O. H., … Bauer, A. (2008). Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. Journal of Neuroscience, 28(52), 14311–14319. Schultz, W., Dayan, P., & Montague, P. R. (1997). A neural substrate of prediction and reward. Science, 275(5306), 1593–1599. Silveri, M. M., Rogowska, J., McCaffrey, A., & Yurgelun-Todd, D. A. (2011). Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during stroop performance. Alcoholism-Clinical and Experimental Research, 35(2), 218–228. Sim, M. E., Lyoo, I. K., Streeter, C. C., Covell, J., Sarid-Segal, O., Ciraulo, D. A., … Renshaw, P. F. (2007). Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology, 32(10), 2229–2237. Skinner, H. A. (1982). The drug abuse screening test. Addictive Behaviors, 7(4), 363–371. Small, D. M., Jones-Gotman, M., & Dagher, A. (2003). Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage, 19(4), 1709–1715. Smith, S. M., & Nichols, T. E. (2009). Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98. Soar, K., Mason, C., Potton, A., & Dawkins, L. (2012). Neuropsychological effects associated with recreational cocaine use. Psychopharmacology, 222(4), 633–643. Solomon, R. L. (1980). The opponent-process theory of acquired motivation: the costs of pleasure and the benefits of pain. American Psychologist, 35(8), 691–712. 175 References Spitz, M. R., Detry, M. A., Pillow, P., Hu, Y. H., Amos, C. I., Hong, W. K., & Wu, X. F. (2000). Variant alleles of the D2 dopamine receptor gene and obesity. Nutrition Research, 20(3), 371–380. Stanek, K. M., Strain, G., Devlin, M., Cohen, R., Paul, R., Crosby, R. D., … Gunstad, J. (2013). Body mass index and neurocognitive functioning across the adult lifespan. Neuropsychology, 27(2), 141–151. Stice, E., Spoor, S., Bohon, C., & Small, D. M. (2008). Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science, 322(5900), 449–452. Stice, E., Yokum, S., Blum, K., & Bohon, C. (2010). Weight gain Is associated with reduced striatal response to palatable food. Journal of Neuroscience, 30(39), 13105– 13109. Streeter, C. C., Terhune, D. B., Whitfield, T. H., Gruber, S., Sarid-Segal, O., Silveri, M. M., … Yurgelun-Todd, D. A. (2008). Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology, 33(4), 827–836. Stroop, J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18, 643–662. Suckling, J., & Bullmore, E. T. (2004). Permutation tests for factorially designed neuroimaging experiments. Human Brain Mapping, 22(3), 193–205. Suckling, J., Davis, M. H., Ooi, C., Wink, A. M., Fadili, J., Salvador, R., … Bullmore, E. T. (2006). Permutation testing of orthogonal factorial effects in a languageprocessing experiment using fMRI. Human Brain Mapping, 27(5), 425–433. Taki, Y., Kinomura, S., Sato, K., Inoue, K., Goto, R., Okada, K., … Fukuda, H. (2008). Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity, 16(1), 119–124. Tanabe, J., Tregellas, J. R., Dalwani, M., Thompson, L., Owens, E., Crowley, T., & Banich, M. (2009). Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biological Psychiatry, 65(2), 160–164. Tanda, G., Pontieri, F. E., & DiChiara, G. (1997). Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu 1 opioid receptor mechanism. Science, 276(5321), 2048–2050. Tanda, G., Pontieri, F. E., Frau, R., & DiChiara, G. (1997). Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. European Journal of Neuroscience, 9(10), 2077–2085. 176 References Tang, D. W., Fellows, L. K., Small, D. M., & Dagher, A. (2012). Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiology & Behavior, 106(3), 317–324. Taylor, J. R., & Robbins, T. W. (1986). 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology, 90(3), 390–397. Thanos, P. K., Volkow, N. D., Freimuth, P., Umegaki, H., Ikari, H., Roth, G., … Hitzemann, R. (2001). Overexpression of dopamine D2 receptors reduces alcohol self-administration. Journal of Neurochemistry, 78(5), 1094–1103. Tiffany, S. T., & Carter, B. L. (1998). Is craving the source of compulsive drug use? Journal of Psychopharmacology, 12(1), 23–30. Tomasi, D., Goldstein, R. Z., Telang, F., Maloney, T., Alia-Klein, N., Caparelli, E. C., & Volkow, N. D. (2007). Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Research, 1171, 83–92. Townshend, J., & Duka, T. (2001). Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology, 157(1), 67–74. Tremblay, L., & Schultz, W. (1999). Relative reward preference in primate orbitofrontal cortex. Nature, 398(6729), 704–708. Tun, P. A., & Lachman, M. E. (2008). Age differences in reaction time and attention in a national telephone sample of adults: education, sex, and task complexity matter. Developmental Psychology, 44(5), 1421–1429. Uhl, G. R., Hall, F. S., & Sora, I. (2002). Cocaine, reward, movement and monoamine transporters. Molecular Psychiatry, 7(1), 21–26. United Nations Office on Drugs and Crime. (2012). World Drug Report 2012. Vienna: United Nations. Vaidya, C. J., Austin, G., Kirkorian, G., Ridlehuber, H. W., Desmond, J. E., Glover, G. H., & Gabrieli, J. D. E. (1998). Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proceedings of the National Academy of Sciences, 95(24), 14494–14499. Verdejo-García, A, & Pérez-García, M. (2007). Ecological assessment of executive functions in substance dependent individuals. Drug and Alcohol Dependence, 90(1), 48–55. Verdejo-Garcia, A. J., Perales, J. C., & Perez-Garcia, M. (2007). Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addictive Behaviors, 32(5), 950–966. 177 References Verdejo-García, Antonio, Bechara, A., Recknor, E. C., & Pérez-García, M. (2006). Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. Journal of the International Neuropsychological Society, 12(3), 405–415. Volkow, N. D., Chang, L., Wang, G. J., Fowler, J. S., Ding, Y. S., Sedler, M., … Pappas, N. (2001). Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. American Journal of Psychiatry, 158(12), 2015–2021. Volkow, N. D., & Fowler, J. S. (2000). Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex, 10(3), 318–325. Volkow, N. D., Fowler, J. S., Wang, G. J., Hitzemann, R., Logan, J., Schlyer, D. J., … Wolf, A. P. (1993). Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse, 14(2), 169–177. Volkow, N. D., Fowler, J. S., Wang, G. J., Telang, F., Logan, J., Jayne, M., … Swanson, J. M. (2010). Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. NeuroImage, 49(3), 2536–2543. Volkow, N. D., Fowler, J. S., Wolf, A. P., Hitzemann, R., Dewey, S., Bendriem, B., … Hoff, A. (1991). Changes in brain glucose-metabolism in cocaine dependence and withdrawal. American Journal of Psychiatry, 148(5), 621–626. Volkow, N. D., Wang, G. J., Begleiter, H., Porjesz, B., Fowler, J. S., Telang, F., … Thanos, P. K. (2006). High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Archives of General Psychiatry, 63(9), 999–1008. Volkow, N. D., Wang, G. J., Fowler, J. S., Gatley, S. J., Ding, Y. S., Logan, J., … Lieberman, J. (1996). Relationship between psychostimulant-induced “high” and dopamine transporter occupancy. Proceedings of the National Academy of Sciences of the United States of America, 93(19), 10388–10392. Volkow, N. D., Wang, G. J., Fowler, J. S., Logan, J., Gatley, S. J., Gifford, A., … Pappas, N. (1999). Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. American Journal of Psychiatry, 156(9), 1440– 1443. Volkow, N. D., Wang, G. J., Fowler, J. S., Logan, J., Gatley, S. J., Hitzemann, R., … Pappas, N. (1997). Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature, 386(6627), 830–833. Volkow, N. D., Wang, G. J., Fowler, J. S., & Telang, F. (2008). Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1507), 3191–3200. 178 References Volkow, N. D., Wang, G. J., Telang, F., Fowler, J. S., Goldstein, R. Z., Alia-Klein, N., … Pradhan, K. (2009). Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity, 17(1), 60–65. Volkow, N. D., Wang, G. J., Telang, F., Fowler, J. S., Logan, J., Childress, A. R., … Wong, C. (2006). Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. Journal of Neuroscience, 26(24), 6583–6588. Volkow, N. D., Wang, G. J., Telang, F., Fowler, J. S., Logan, J., Jayne, M., … Wong, C. (2007). Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. Journal of Neuroscience, 27(46), 12700–12706. Volkow, N. D., Wang, G. J., Telang, F., Fowler, J. S., Thanos, P. K., Logan, J., … Pradhan, K. (2008). Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. NeuroImage, 42(4), 1537–1543. Walther, K., Birdsill, A. C., Glisky, E. L., & Ryan, L. (2010). Structural brain differences and cognitive functioning related to body mass index in older females. Human Brain Mapping, 31(7), 1052–1064. Wang, G. J., Volkow, N. D., Fowler, J. S., Cervany, P., Hitzemann, R. J., Pappas, N. R., … Felder, C. (1999). Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sciences, 64(9), 775–784. Wang, G. J., Volkow, N. D., Logan, J., Pappas, N. R., Wong, C. T., Zhu, W., … Fowler, J. S. (2001). Brain dopamine and obesity. Lancet, 357(9253), 354–357. Wang, G. J., Volkow, N. D., Thanos, P. K., & Fowler, J. S. (2004). Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. Journal of Addictive Diseases, 23(3), 39–53. Weise, C. M., Thiyyagura, P., Reiman, E. M., Chen, K., & Krakoff, J. (2013). Fat-free body mass but not fat mass is associated with reduced gray matter volume of cortical brain regions implicated in autonomic and homeostatic regulation. NeuroImage, 64, 712–721. Wexler, B. E., Gottschalk, C. H., Fulbright, R. K., Prohovnik, I., Lacadie, C. M., Rounsaville, B. J., & Gore, J. C. (2001). Functional magnetic resonance imaging of cocaine craving. American Journal of Psychiatry, 158(1), 86–95. Whelan, R., Conrod, P. J., Poline, J. B., Lourdusamy, A., Banaschewski, T., Barker, G. J., … Garavan, H. (2012). Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature Neuroscience, 15(6), 920–925. Wise, R. A. (2004). Dopamine, learning and motivation. Nature Reviews: Neuroscience, 5(6), 483–494. Wise, R. A., & Bozarth, M. A. (1985). Brain mechanisms of drug reward and euphoria. Psychiatic Medicine, 3(4), 445–460. 179 References Wise, R. A., & Rompre, P. P. (1989). Brain dopamine and reward. Annual Review of Psychology, 40, 191–225. Wise, R. A., Spindler, J., DeWit, H., & Gerberg, G. J. (1978). Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science, 201(4352), 262–264. Woolverton, W. L., & Anderson, K. G. (2006). Effects of delay to reinforcement on the choice between cocaine and food in rhesus monkeys. Psychopharmacology, 186(1), 99–106. Wrase, J., Schlagenhauf, F., Kienast, T., Wüstenberg, T., Bermpohl, F., Kahnt, T., … Heinz, A. (2007). Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage, 35(2), 787–794. Wyvell, C. L., & Berridge, K. C. (2001). Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. Journal of Neuroscience, 21(19), 7831–7840. Yin, H. H., & Knowlton, B. J. (2006). The role of the basal ganglia in habit formation. Nature Reviews: Neuroscience, 7(6), 464–476. Yin, H. H., Knowlton, B. J., & Balleine, B. W. (2004). Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. European Journal of Neuroscience, 19(1), 181–189. Yin, H. H., Ostlund, S. B., & Balleine, B. W. (2008). Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. European Journal of Neuroscience, 28(8), 1437–1448. Yokum, S., Ng, J., & Stice, E. (2012). Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. International journal of obesity (2005), 36(5), 656–64. Zhang, Y., Brady, M., & Smith, S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE transactions on medical imaging, 20(1), 45–57. Ziauddeen, H., Farooqi, S., & Fletcher, P. C. (2012). Obesity and the brain: how convincing is the addiction model? Nature Reviews. Neuroscience, 13(4), 279–286. Ziauddeen, H., & Fletcher, P. C. (2013). Is food addiction a valid and useful concept? Obesity Reviews, 14(1), 19–28. Zuckerman, M., Eysenck, S., & Eysenck, H. J. (1978). Sensation seeking in England and America: cross-cultural, age, and sex comparisons. Journal of Consulting and Clinical Psychology, 46(1), 139–149. 180 References Zysset, S., Muller, K., Lohmann, G., & von Cramon, D. Y. (2001). Color-word matching stroop task: Separating interference and response conflict. NeuroImage, 13(1), 29– 36. 181 Appendices Appendices Appendix A. Supplemental Information for Chapter 2 Table S2.1. As sex, education, smoking status, depression scores and alcohol use all significantly differed between participant groups, largely driven by the stimulantdependent individuals, we included these as covariates in the general linear model multivariate regression analysis. However, there were no differences in the results both with and without these covariates (Miller & Chapman, 2001). As these variables seem to be somewhat intrinsically linked to drug addiction — nicotine dependence, alcohol abuse and depression commonly occurring as co-morbidities in the disorder — we thought it prudent to report the results without using these variables as covariates. However, we report the results including covariates in the model, as there were slight discrepancies. Covariates included F(2,129) P Covariates excluded F(2,135) P Behavioural analysis Congruent RT 3.74 0.026* 7.243 Incongruent RT 3.86 0.024* 4.452 Interference 0.96 0.387 0.797 Errors 2.92 0.057 1.915 Imaging analysis: First level Left precentral / middle frontal gyrus 0.14 0.873 0.330 Left inferior frontal gyrus 0.74 0.482 0.261 Right caudate 0.86 0.424 0.542 Right rolandic operculum 0.16 0.851 0.001 Behavioural analysis: Second level Right insula / rolandic operculum 16.94 <0.001* 18.373 Left medial superior frontal gyrus 11.85 <0.001* 12.094 Behavioural analysis: Second level: Inferior frontal gyrus mask Left inferior frontal gyrus 8.96 <0.001* 11.981 *Significant group difference at p<0.05 182 0.001* 0.013* 0.450 0.151 0.719 0.771 0.583 0.999 <0.001* <0.001* <0.001* Appendices Appendix B. Supplemental Information for Chapter 3 Table S3.1. Behavioural results for the cocaine-word Stroop, comparing recreational cocaine users, stimulant-dependent individuals (SDI) and healthy control volunteers using ANCOVA models with Bonferroni post-hoc corrections. Dependent stimulant users were significantly more impaired on the task, demonstrated via greater interference scores, higher response latencies and more errors committed. Recreational users and controls did not differ behaviourally on any measure. Covariates in the analysis include age, gender, education, smoking status, BDI-II depression and AUDIT alcohol scores. Control Recreational Median (SD) Median (SD) Behavioural Results Cocaine RT* 687.05(144.35)a 655.00(85.25)c Neutral RT* 669.37(138.45)a 677.94(101.40) Interference 17.68(97.90) -22.94(74.96) Cocaine word 0.70(0.91)a 0.61(0.70)c errors* Neutral word errors* 1.41(3.26)a 1.31(1.26) SDI Median (SD) F/X2 P 874.44(222.20)ac 784.11(187.27)a 90.32(161.07) 6.57 3.67 3.02 0.002 0.029 0.053 2.32(2.25)ac 23.01 <0.001 2.53(3.27)a 6.62 0.036 *Significant group difference at p<0.05 X2 Kruskal-Wallis Test a c Significance between SDI and Control groups in Bonferroni post-hoc test at p<0.01. Significance between SDI and Recreational groups in Bonferroni post-hoc test at p<0.01. 183 Appendices Table S3.2. Peak activation voxels for clusters identified during a first-level whole-brain imaging analysis amongst all participants, contrasting differences in activation during cocaine versus neutral word trials. Significance set a p<0.05 family-wise error correction for multiple comparisons. Coordinates listed are in MNI standard space. First-level analysis Contrast activation areas Cluster 1 Left gyrus rectus, left middle frontal orbital, right gyrus rectus Cluster 2 Left inferior frontal gyrus – pars triangularis, left inferior frontal gyrus – pars orbitalis, left insula Cluster 3 Left superior medial frontal gyrus, right superior medial frontal gyrus, left anterior cingulum, right anterior cingulum Cluster 4 Left angular gyrus, left middle temporal gyrus Cluster 5 Right precuneus, right cuneus, right superior parietal lobe, right superior occipital lobe Cluster 6 Right inferior parietal lobe, right postcentral sulcus, right supramarignal gyrus, right superior parietal lobe Brodmann Area Cluster size (voxels) Max F Peak MNI coordinates 11 83 3.51 -6, 50, -16 13, 45, 47 153 2.70 -50, 36, -2 9, 10, 32 80 2.68 6, 54, 26 39 93 2.33 -52, -58, 24 7 98 -2.78 10, -74, 48 1, 2, 40 84 -2.59 50, -48, 48 184 Appendices Table S3.3. Mean activation levels for second-level fMRI general linear model omnibus contrasts comparing brain activation between groups during the cocaine–neutral word contrast, both with and without a small-volume correction mask of the inferior frontal gyrus. Coordinates listed are in MNI standard space. Cluster significance set a p<0.05 family-wise error correction for multiple comparisons. Post-hoc group comparisons made using ANCOVA models, controlling for age, gender, years of education, smoking status, BDI-II depression and AUDIT alcohol scores, with Bonferroni correction p<0.05. Mean Activation Levels Control Recreational SDI F P GLM activation areas Cluster1 – 169 voxels* BA 10, 11, 32 R middle orbitofrontal cortex R anterior cingulate cortex R superior med frontal lobe R superior orbitofrontal cortex Cluster 2 – 111 voxels* BA 31 R angular gyrus R posterior cingulate cortex GLM IFG activation areas Cluster1 – 27 voxels* BA 44 R IFG pars opercularis R rolandic operculum Cluster 2 – 21 voxels R IFG pars triangularis R insula Cluster 2 – 31 voxels* BA 13, 44 R IFG pars opercularis R insula R rolandic operculum 4.32b -126.03bc 102.43c 10.297 <.001 0.89b -111.23bc 37.93c 10.649 <.001 -15.64ab 7.56b 11.24a 9.761 <0.001 -0.48b -19.36bc 16.60c 8.129 <0.001 -11.79 -27.77c 11.58c 5.604 0.005 *Significant group difference at FWE p<0.05 a Significance between SDI and Control groups in Bonferroni post-hoc test at p<0.01 b Significance between Recreational and Control in Bonferroni post-hoc test at p<0.01 c Significance between SDI and Recreational in Bonferroni post-hoc test at p<0.01 185 Appendices A) IFG Interference Cluster 1 IFG Interference Cluster 2 IFG Interference Cluster 3 B) 186 Appendices Figure S3.1. A) A regression of behavioural interference scores onto fMRI imaging contrasts comparing cocaine and neutral words resulted in a positive correlation between interference on the task and activation in three clusters in the right inferior frontal gyrus (IFG). Greater activity in the right IFG related to increased impairment on the task, with greater distraction to the cocaine-salient words. This effect was largely driven by the stimulant-dependent individuals, who had significantly more activation in this region compared with recreational cocaine users. B) Between group comparison of BOLD activation in clusters in the right IFG that correlated with interference scores on the cocaine Stroop task. Recreational cocaine users again showed less activation than dependent users, though this was only significant in one cluster using ANCOVA models, controlling for age, gender, years of education, smoking status, BDI-II depression and AUDIT alcohol scores, with Bonferroni post-hoc correction p<0.05. 187 Appendices Appendix C. Supplemental Information for Chapter 4 Table S4.1. Demographic information for sample used in the Monetary Incentive Delay task of 35 stimulant-dependent individuals, 40 of their non-dependent biological siblings, 22 recreational users of cocaine, and 43 unrelated healthy control volunteers. Demographics Age* Gender (n male:%)* NART (score)* Education (years)* BDI-II (total score)* AUDIT (total score)* Tobacco use (%)* Cannabis use (%)* Control Mean (SD) Sibling Mean (SD) Recreational Mean (SD) Dependent Mean SD) F / χ2 P 32.44 (8.90) 28 (65.1%) 111.60 (1.29) 12.60 (1.94) 2.05 (2.21) 3.32 (2.36) 26 (60.5%) 10 (23.3%) 31.77 (8.48) 19 (47.5%) 108.89 (8.33) 12.00 (1.95) 4.98 (6.09) 4.12 (4.78) 37 (92.5%) 30 (75.0%) 27.50 (6.16) 11 (50.0%) 115.75 (5.40) 13.41 (1.79) 3.68 (4.42) 5.73 (1.61) 19(86.4%) 21(95.5%) 34.48 (7.83) 33 (94.3%) 110.48 (7.85) 11.63 (1.75) 17.91 (12.07) 13.76 (11.82) 34(97.1%) 35(100%) 3.38 20.61 3.43 4.78 36.36 19.16 32.90 64.69 0.020 <0.001 0.019 0.003 <0.001 <0.001 <0.001 <0.001 National Adult Reading Test (NART), Beck Depression Inventory (BDI-II), Alcohol-Use Disorders Identification Test (AUDIT) 188 Appendices Figure S4.1. Differences in subjective ratings for a 10 pence piece, judged by selfreported likelihood to pick the coin up off the ground. Sibling participants reported themselves as less likely to pick up the coin compared with their stimulant-dependent brothers and sisters, though this did not survive more stringent Bonferroni post-hoc corrections. This decrease in reward valuation was later reflected in their neural activation during the Monetary Incentive Delay task, particularly in functional activity in the right supplementary motor area during anticipation of a money trial, which significantly correlated with this subjective 10p rating. 189 Appendices Figure S2. Difference between non-drug-using and drug-using participant groups in BOLD activity in the right orbitofrontal gyrus during feedback for successful versus unsuccessful trials during the money condition. The sibling and control participants displayed significantly more activation than both the dependent and recreational cocaine users in this area at FWE p<0.05, though the groups did not differ with Bonferroni posthoc tests for directionality. 190 Appendices Figure S3. Difference between drug-using and non-drug-using groups in BOLD activity in the right orbitofrontal gyrus during anticipation of drug versus neutral cues. The sibling participants displayed significantly less activation than both the dependent and recreational cocaine users. Control individuals did not differ from either group. 191 Appendices Appendix D. Supplemental Information for Chapter 6 Table S6.1. Voxel coordinates of orbitofrontal clusters associated with either body mass index (BMI) or years of cocaine use in control and cocaine-dependent individuals. Regions overlapping, or nearly overlapping – no more than four voxels away in any direction, are highlighted as follows: Control (con) BMI and cocaine-dependent (coc) BMI Control BMI and cocaine-dependent years of use (YOU) Cocaine-dependent BMI and YOU 192 Appendices Cluster 21 22 23 24 25 26 Con BMI 1 56 voxels -4,40,-30 -4,40,-28 -6,56,-28 -2,42,-26 4,44,-26 -2,52,-26 -6,56,-26 -4,32,-24 2,34,-24 6,36,-24 -4,40,-24 2,40,-24 -8,56,-24 2,-46,-24 8,54,-24 -2,28,-22 2,34,-22 6,36,-22 2,44,-22 -4,42,-22 -2,46,-22 14,48,-22 4,52,-22 6,58,-22 -6,56,-22 8,62,-20 22,42,-20 -4,42,-20 16,48,-20 Con BMI 2 223 voxels Con BMI 3 45 voxels Con BMI 4 23 voxels Coc BMI 1 93 voxels Coc BMI 2 81 voxels Coc YOU 1 269 voxels Coc YOU 2 192 voxels -6,42,-28 -2,42,-26 4,44,-26 -2,52,-26 -6,56,-26 -4,32,-24 2,34,-24 6,36,-24 -4,40,-24 2,40,-24 -8,56,-24 2,-46,-24 8,54,-24 -2,28,-22 2,34,-22 6,36,-22 2,44,-22 -4,42,-22 -2,46,-22 14,48,-22 4,52,-22 6,58,-22 -6,56,-22 8,62,-20 22,42,-20 -4,42,-20 16,48,-20 193 -2,22,-26 16,24,-26 -8,42,-26 16,24,-26 -8,42,-26 0,26,-24 0,38,-24 18,24,-24 -12,42,-24 -14,46,-24 18,24,-24 -12,42,-24 -14,46,-24 4,34,-22 2,38,-22 2,42,-22 16,60,-22 4,34,-22 2,38,-22 2,42,-22 16,60,-22 24,26,-22 28,32,-22 -20,32,-22 18,34,-22 -24,34,-22 -12,42,-22 -16,46,-22 -12,50,-22 -12,56,-22 24,26,-22 28,32,-22 -20,32,-22 18,34,-22 -24,34,-22 -12,42,-22 -16,46,-22 -12,50,-22 -12,56,-22 4,40,-20 12,60,-20 4,40,-20 12,60,-20 24,26,-20 -20,26,-20 30,32,-20 -24,34,-20 24,26,-20 -20,26,-20 30,32,-20 -24,34,-20 Appendices -2,46,-20 -6,56,-20 -2,46,-20 -6,56,-20 27 2,28,-18 4,46,-18 14,54,-18 -28,54,-18 4,54,-18 -4,56,-18 -12,60,-18 10,62,-18 -20,64,-18 2,28,-18 4,46,-18 14,54,-18 -28,54,-18 4,54,-18 -4,56,-18 -12,60,-18 10,62,-18 -20,64,-18 2,28,-18 4,46,-18 14,54,-18 -28,54,-18 4,54,-18 -4,56,-18 -12,60,-18 10,62,-18 -20,64,-18 4,34,-18 16,56,-18 4,34,-18 16,56,-18 28 -2,52,-16 24,44,-16 18,54,-16 -28,54,-16 -18,52,-16 -20,62,-16 4,60,-16 -2,52,-16 24,44,-16 18,54,-16 -28,54,-16 -18,52,-16 -20,62,-16 4,60,-16 -2,52,-16 24,44,-16 18,54,-16 -28,54,-16 -18,52,-16 -20,62,-16 4,60,-16 4,28,-16 4,44,-16 24,48,-16 12,60,-16 4,28,-16 4,44,-16 24,48,-16 12,60,-16 29 -8,46,-14 -18,48,-14 18,54,-14 -2,48,-14 4,52,-14 -20,62,-14 -8,46,-14 -18,48,-14 18,54,-14 -2,48,-14 4,52,-14 -20,62,-14 -8,46,-14 -18,48,-14 18,54,-14 -2,48,-14 4,52,-14 -20,62,-14 4,28,-14 24,48,-14 22,56,-14 2,56,-14 12,60,-14 4,28,-14 24,48,-14 22,56,-14 2,56,-14 12,60,-14 194 20,40,-20 -16,46,-20 -16,52,-20 22,18,-18 30,24,-18 -22,26,-18 30,32,-18 18,32,-18 -30,38,-18 20,36,-18 28,42,-18 -18,44,-18 -16,52,-18 -12,60,-18 24,24,-16 -22,26,-16 26,42,-16 -16,30,-16 16,32,-16 40,36,-16 -26,40,-16 -22,50,-16 -28,48,-16 24,24,-14 -40,26,-14 -24,30,-14 20,34,-14 -28,32,-14 40,36,-14 -28,36,-14 -34,36,-14 20,40,-20 -16,46,-20 -16,52,-20 22,18,-18 30,24,-18 -22,26,-18 30,32,-18 18,32,-18 -30,38,-18 20,36,-18 28,42,-18 -18,44,-18 -16,52,-18 -12,60,-18 24,24,-16 -22,26,-16 26,42,-16 -16,30,-16 16,32,-16 40,36,-16 -26,40,-16 -22,50,-16 -28,48,-16 24,24,-14 -40,26,-14 -24,30,-14 20,34,-14 -28,32,-14 40,36,-14 -28,36,-14 -34,36,-14 Appendices 30 20,52,-12 -18,48,-12 -24,46,-12 -28,56,-12 -18,60,-12 20,52,-12 -18,48,-12 -24,46,-12 -28,56,-12 -18,60,-12 4,32,-12 24,56,-12 12,58,-12 4,32,-12 24,56,-12 12,58,-12 31 -16,48,-10 20,52,-10 24,62,-10 -16,48,-10 20,52,-10 24,62,-10 4,28,-10 8,34,-10 16,58,-10 26,60,-10 4,28,-10 8,34,-10 16,58,-10 26,60,-10 32 22,58,-8 6,36,-8 24,60,-8 6,36,-8 24,60,-8 33 6,40,-6 34 4,42,-4 195 -20,56,-14 24,44,-14 -38,28,-12 -20,28,12 40,32,-12 20,34,-12 -28,32,-12 30,32,-12 36,34,-12 -26,44,-12 38,38,-12 30,38,-12 -18,44,-12 -30,42,-10 40,30,-10 28,34,-10 36,40,-10 -28,36,-10 30,38,-10 -16,48,-10 -30,52,-10 36,36,-8 -34,38,-8 -30,38,-8 38,42,-8 38,30,-6 -30,40,-6 -32,36,-4 -20,56,-14 24,44,-14 -38,28,-12 -20,28,12 40,32,-12 20,34,-12 -28,32,-12 30,32,-12 36,34,-12 -26,44,-12 38,38,-12 30,38,-12 -18,44,-12 -30,42,-10 40,30,-10 28,34,-10 36,40,-10 -28,36,-10 30,38,-10 -16,48,-10 -30,52,-10 36,36,-8 -34,38,-8 -30,38,-8 38,42,-8 38,30,-6 -30,40,-6 Appendices Appendix E. Ersche KD, Jones PS, Williams GB, Smith DG, Bullmore ET, Robbins TW. (2013). Distinctive personality traits and neural correlates associated with stimulant drug use versus risk of stimulant dependence. Biological Psychiatry, 74, 137-144. 196