Example 1
advertisement

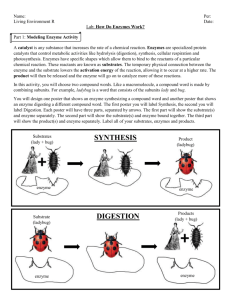
Potato Peroxidase Lab Conclusion The purpose of this lab was to determine the answer to the question, "how does temperature and pH of peroxidase affect its ability to perform its function and break down hydrogen peroxide?” Enzymes are proteins that catalyze reactions, which means they lower the activation energy so the reaction can happen faster. A substrate is the material upon which an enzyme acts. In the case of this lab, the substrate was hydrogen peroxide. Peroxidase is an enzyme that speeds up the chemical breakdown of hydrogen peroxide into oxygen gas and water. In this lab, the control was the room temperature, untreated potato slice. The independent variable was the conditions we applied to the potato slices, which included temperature (heated and frozen) and pH level (acid and base). The dependent variable was the rate of the decomposition reaction of hydrogen peroxide into water and oxygen, which was an indicator of the performance of the enzyme peroxidase. The hypothesis was that heat and a basic environment will speed up the chemical breakdown of hydrogen peroxide by peroxidase. This was predicted because it was thought that heat would increase the speed of the molecules, thus increasing the probability of collisions and the rate of reaction. This hypothesis was rejected. It was actually found that a neutral environment and a frozen temperature sped up the reaction. The neutral potato at room temperature reacted and foamed the fastest, followed by the frozen potato slice. In the acid treated, base treated, and heated potato slices, there was little to no reaction. It seems that the extreme heat of boiling and the extremely acidic and basic environments of the HCl and NaOH denatured the protein in the enzyme, altering its 3D structure and therefore altering its function. One source of error in this experiment is the fact that there might have been some parts of the acid and basic potato that weren't treated by the HCl and NaOH. When the hydrogen peroxide was dripped on the potato, if any landed on the untreated section it could've resulted in a false positive for if peroxidase still performed its function. For example, the rate of reaction on the basic potato was recorded as slow, because there were a few small bubbles that appeared, but this was likely a result of the hydrogen peroxide reacting with a spot that the NaOH missed. Another source of error is the fact that the heated potato was not actually hot when we tested it. It had been boiled previously, but then allowed to cool. The actual temperature, not the previous condition of heat, may have affected the rate of the reaction of the peroxidase enzyme when we added the hydrogen peroxide. This lab could be taken further by applying it to real world situations like the human body. This lab can be an indicator of the necessary conditions in humans for enzymes to function properly. It is also illustrative of how different variations of those conditions, like pH or temperature, can influence the function and effectiveness of the enzymes. In humans, the enzyme that performed the same function is catalase, an enzyme in the liver that breaks down hydrogen peroxide into water and oxygen. By looking at this lab and taking it further, any problems with that enzyme in a human could be understood to possibly be a result of too high a temperature (which would denature it) or a drastic change in pH which would also alter its function. Without understanding of the conditions that can impact an enzyme, it is more difficult to diagnose any issues someone might be having with catalase, or any enzyme.