AP Chemistry Equilibrium Exam Problems
advertisement
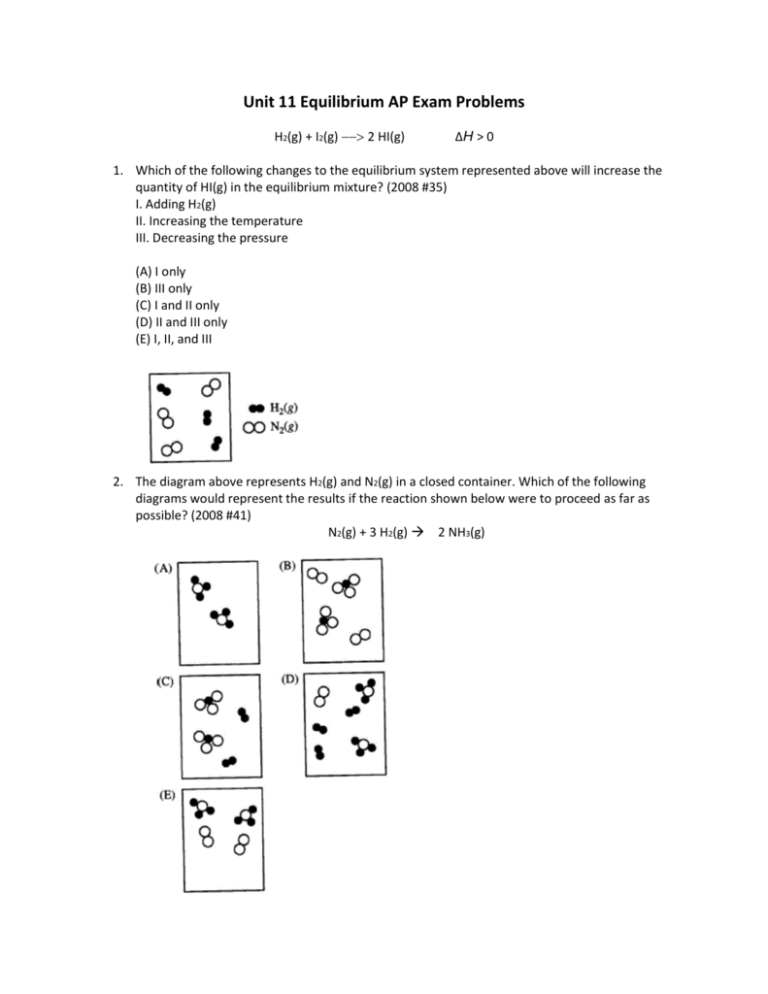
Unit 11 Equilibrium AP Exam Problems H2(g) + I2(g) 2 HI(g) ΔH > 0 1. Which of the following changes to the equilibrium system represented above will increase the quantity of HI(g) in the equilibrium mixture? (2008 #35) I. Adding H2(g) II. Increasing the temperature III. Decreasing the pressure (A) I only (B) III only (C) I and II only (D) II and III only (E) I, II, and III 2. The diagram above represents H2(g) and N2(g) in a closed container. Which of the following diagrams would represent the results if the reaction shown below were to proceed as far as possible? (2008 #41) N2(g) + 3 H2(g) 2 NH3(g) 3. The reaction mixture represented above is at equilibrium at 298 K, and the molar concentrations are [X] = 2.0 M, [Y] = 0.5 M, and [Z] = 4.0 M. What is the value of the equilibrium constant for the reaction at 298 K? (2008 #55) (A) 0.50 (B) 2.0 (C) 4.0 (D) 16 (E) 32 4. The diagram above represents a mixture of NO2(g) and N2O4(g) in a 1.0 L container at a given temperature. The two gases are in equilibrium according to the equation 2 NO2(g) N2O4(g). Which of the following must be true about the value of the equilibrium constant for the reaction at this temperature? (2008 #59) (A) K = 0 (B) 0 < K < 1 (C) K = 1 (D) K > 1 (E) There is not enough information to determine the relative value of K. 5. The figure above shows two closed containers. Each contains the same volume of acetone in equilibrium with its vapor at the same temperature. The vapor pressure of acetone is (2008 #73) (A) higher in container 1 because the surface are of the liquid is greater (B) higher in container 1 because the volume of vapor is greater (C) lower in container 1 because the level of the liquid is lower (D) the same in both containers because the volume of the liquid is the same (E) the same in both containers because the temperature is the same H2(g) + Br2(g) 2 HBr(g) 6. At a certain temperature, the value of the equilibrium constant, K, for the reaction represented above is 2.0 × 105. What is the value of K for the reverse reaction at the same temperature? (2002 #42) (A) -2.0 × 10-5 (B) 5.0 × 10-6 (C) 2.0 × 10-5 (D) 5.0 × 10-5 (E) 5.0 × 10-4 7. In a saturated solution of Zn(OH)2 at 25 °C, the value of [OH-] is 2.0 × 10-6 M. What is the value of the solubility-product constant, Ksp, for Zn(OH)2 at 25 °C? (2002 #75) (A) 4.0 × 10-18 (B) 8.0 × 10-18 (C) 1.6 × 10-17 (D) 4.0 × 10-12 (E) 2.0 × 10-6 2NO(g) + O2(g) 2 NO2(g) H < 0 8. Which of the following changes alone would cause a decrease in the value of Keq for the reaction represented above? (1999 #54) A) Decreasing the temperature B) Increasing the temperature C) Decreasing the volume of the reaction vessel D) Increasing the volume of the reaction vessel (E) Adding a catalyst PCl3(g) + Cl2(g) <===> PCl5(g) + energy 9. Some PCl3 and Cl2 are mixed in a container at 200 °C and the system reaches equilibrium according to the equation above. Which of the following causes an increase in the number of moles of PCl5 present at equilibrium? (1994 #48) I. Decreasing the volume of the container II. Raising the temperature III. Adding a mole of He gas at constant volume (A) I only (B) II only (C) I and III only (D) II and III only (E) I, II, and III 4 HCl(g) + O2(g) <===> 2 Cl2(g) + 2 H2O(g) 10. Equal numbers of moles of HCl and O2 in a closed system are allowed to reach equilibrium as represented by the equation above. Which of the following must be true at equilibrium? (1999 #51) I. [HCl] must be less than [Cl2]. II. [O2] must be greater than [HCl]. III. [Cl2] must equal [H2O]. (A) I only (B) II only (C) I and III only (D) II and III only (E) I, II, and III 2 SO2(g) + O2(g) <===> 2 SO3(g) 11. When 0.40 mole of SO2 and 0.60 mole of O2 are placed in an evacuated 1.00-liter flask, the reaction represented above occurs. After the reactants and the product reach equilibrium and the initial temperature is restored, the flask is found to contain 0.30 mole of SO3. Based on these results, the equilibrium constant, Kc for the reaction is (1994 #73) (A) 20. (B) 10. (C) 6.7 (D) 2.0 (E) 1.2 Answers to Equilibrium Multiple Choice Problems 1. C 2. E 3. E 4. B 5. E 6. B 7. A 8. B 9. A 10. D 11. A











