CLASSWORK FEEDBACK: CRUDE OIL AND FUELS, USEFUL

CLASSWORK FEEDBACK: CRUDE OIL AND FUELS, USEFUL SUBSTANCES FROM OIL
TASK Complete the ‘ Do I understand topic ’ column using :
✓
(Yes),
✗
(No), or ?
(Unsure).
Select question tasks to complete that will total to 10 points (e.g. three task 1s + two task 2s + one task 3= 10 points).
ANY TASK COMBINATION is allowed-but total score must be at least 10 pts.
Do questions on your weakest topics , but attempting ALL questions is recommended (maximum score is 42 points)
Do I understand topic Question Task 1
(ONE point)
Question Task 2
(TWO points)
Question Task 3
(THREE points)
Crude oil composition
Teacher’s view
Student’s view
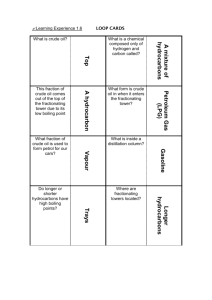
What is a mixture? What is a hydrocarbon? Is crude oil a mixture of liquid hydrocarbons with different boiling points?
List 4 fractions from crude oil in order of small-to-large molecule size?
Fractional distillation of crude oil
Alkanes
Alkenes
What is the difference between boiling and condensing?
Does this occur at the same temperature for a liquid?
Draw the structural formula for ethane (C2H6)
Draw the structural formula for ethene (C2H4)
Do small molecule size hydrocarbons have low or high boiling points?
Do large molecules size hydrocarbons condense at the top or bottom of the tower?
How many hydrogen atoms occur in a molecule of propane?
What is different about the carbon-carbon bond between an alkane and an alkene and how can you test for this difference?
What is a catalyst?
Why is the fractionating tower deliberately cool at the top and hot at the bottom?
What is the general formula for all alkanes?
What is the general formula for all alkenes?
Cracking oil
Burning fuels
Cracking is a type of thermal decomposition that uses heat and a catalyst- True or False?
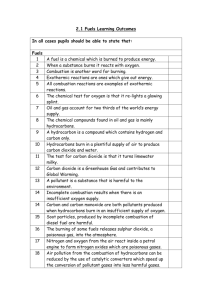
What two products are produced by burning methane with oxygen?
Environmental effects of plastic bags and
All plastic bags are biodegradable (broken down by microbes)-True of False? containers
Assessment of Class book quality by Teacher Never
Incomplete homework
Unattached or incomplete worksheets
Untidy work (e.g. not dated, spelling errors, scribbles, untidy etc.)
How can you test for the products produced from burning methane with oxygen?
Poly(ethene) polymers are made by joining together many small molecules of ethene-True of False?
Occasional
Why is it useful to be able to crack larger hydrocarbons into smaller hydrocarbons?
Which pollutants cause acid rain, global warming and global dimming?
List two advantages and two disadvantages for recycling plastic bags and containers?
Frequent
EXTENSION WORK : http://ed.ted.com/on/HGaafIiY (fractional distillation oil), http://ed.ted.com/on/OmkI7cEx
(alkanes/alkenes), http://ed.ted.com/on/oaPgWq8X (catalytic cracking), http://ed.ted.com/on/NgfHUQ67
(combustion of hydrocarbons)
STUDENT FEEDBACK- to complete AFTER finishing the tasks
What went well?
What could I improve on and how?
Teacher’s response to student feedback
Signature
Date
CLASSWORK FEEDBACK: CRUDE OIL AND FUELS, USEFUL SUBSTANCES FROM OIL
TASK Complete the ‘ Do I understand topic ’ column using :
✓
(Yes),
✗
(No), or ?
(Unsure).
Select question tasks to complete that will total to 10 points (e.g. three task 1s + two task 2s + one task 3= 10 points).
ANY TASK COMBINATION is allowed-but total score must be at least 10 pts.
Do questions on your weakest topics , but attempting ALL questions is recommended (maximum score is 42 points)
Do I understand topic Question Task 1
(ONE point)
Question Task 2
(TWO points)
Question Task 3
(THREE points)
Crude oil composition
Teacher’s view
Student’s view
What is a mixture? What is a hydrocarbon? Is crude oil a mixture of liquid hydrocarbons with different boiling points?
List 4 fractions from crude oil in order of small-to-large molecule size?
Fractional distillation of crude oil
Alkanes
Alkenes
What is the difference between boiling and condensing?
Does this occur at the same temperature for a liquid?
Draw the structural formula for ethane (C2H6)
Draw the structural formula for ethene (C2H4)
Do small molecule size hydrocarbons have low or high boiling points?
Do large molecules size hydrocarbons condense at the top or bottom of the tower?
How many hydrogen atoms occur in a molecule of propane?
What is different about the carbon-carbon bond between an alkane and an alkene and how can you test for this difference?
What is a catalyst?
Why is the fractionating tower deliberately cool at the top and hot at the bottom?
What is the general formula for all alkanes?
What is the general formula for all alkenes?
Cracking oil
Burning fuels
Cracking is a type of thermal decomposition that uses heat and a catalyst- True or False?
What two products are produced by burning methane with oxygen?
Environmental effects of plastic bags and
All plastic bags are biodegradable (broken down by microbes)-True of False? containers
Assessment of Class book quality by Teacher Never
Incomplete homework
Unattached or incomplete worksheets
Untidy work (e.g. not dated, spelling errors, scribbles, untidy etc.)
How can you test for the products produced from burning methane with oxygen?
Poly(ethene) polymers are made by joining together many small molecules of ethene-True of False?
Occasional
Why is it useful to be able to crack larger hydrocarbons into smaller hydrocarbons?
Which pollutants cause acid rain, global warming and global dimming?
List two advantages and two disadvantages for recycling plastic bags and containers?
Frequent
EXTENSION WORK : http://ed.ted.com/on/HGaafIiY (fractional distillation oil), http://ed.ted.com/on/OmkI7cEx
(alkanes/alkenes), http://ed.ted.com/on/oaPgWq8X (catalytic cracking), http://ed.ted.com/on/NgfHUQ67
(combustion of hydrocarbons)
STUDENT FEEDBACK- to complete AFTER finishing the tasks
What went well?
What could I improve on and how?
Teacher’s response to student feedback
Signature
Date