Data Linkage Variable Request Form
advertisement
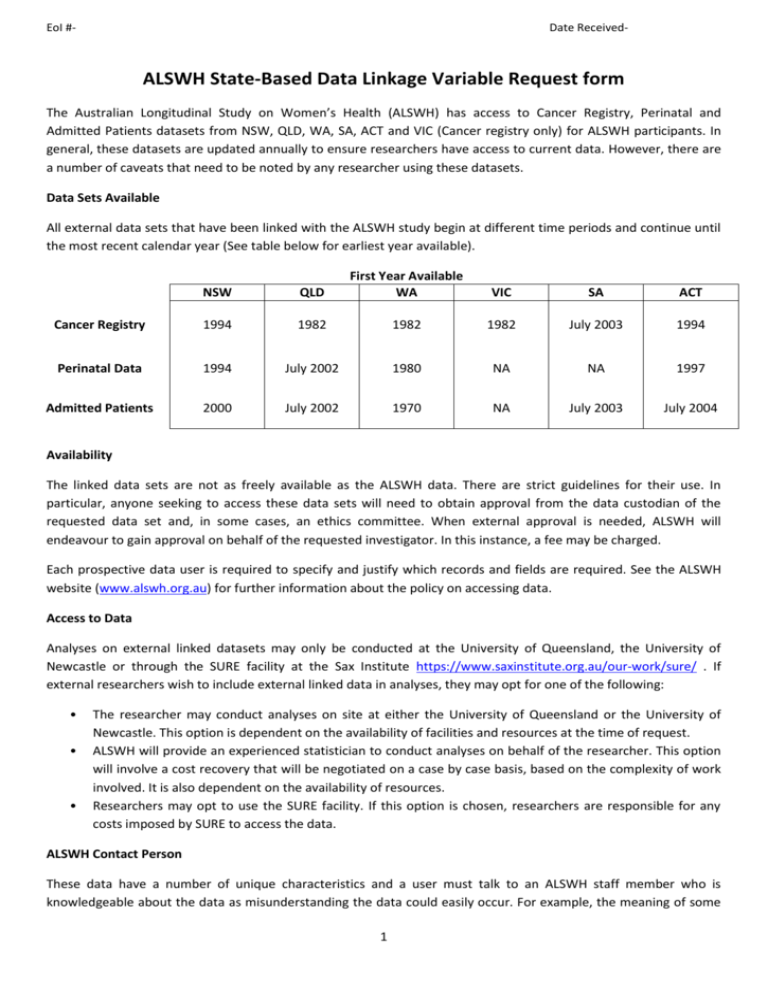
EoI #- Date Received- ALSWH State-Based Data Linkage Variable Request form The Australian Longitudinal Study on Women’s Health (ALSWH) has access to Cancer Registry, Perinatal and Admitted Patients datasets from NSW, QLD, WA, SA, ACT and VIC (Cancer registry only) for ALSWH participants. In general, these datasets are updated annually to ensure researchers have access to current data. However, there are a number of caveats that need to be noted by any researcher using these datasets. Data Sets Available All external data sets that have been linked with the ALSWH study begin at different time periods and continue until the most recent calendar year (See table below for earliest year available). NSW QLD First Year Available WA VIC SA ACT Cancer Registry 1994 1982 1982 1982 July 2003 1994 Perinatal Data 1994 July 2002 1980 NA NA 1997 Admitted Patients 2000 July 2002 1970 NA July 2003 July 2004 Availability The linked data sets are not as freely available as the ALSWH data. There are strict guidelines for their use. In particular, anyone seeking to access these data sets will need to obtain approval from the data custodian of the requested data set and, in some cases, an ethics committee. When external approval is needed, ALSWH will endeavour to gain approval on behalf of the requested investigator. In this instance, a fee may be charged. Each prospective data user is required to specify and justify which records and fields are required. See the ALSWH website (www.alswh.org.au) for further information about the policy on accessing data. Access to Data Analyses on external linked datasets may only be conducted at the University of Queensland, the University of Newcastle or through the SURE facility at the Sax Institute https://www.saxinstitute.org.au/our-work/sure/ . If external researchers wish to include external linked data in analyses, they may opt for one of the following: • • • The researcher may conduct analyses on site at either the University of Queensland or the University of Newcastle. This option is dependent on the availability of facilities and resources at the time of request. ALSWH will provide an experienced statistician to conduct analyses on behalf of the researcher. This option will involve a cost recovery that will be negotiated on a case by case basis, based on the complexity of work involved. It is also dependent on the availability of resources. Researchers may opt to use the SURE facility. If this option is chosen, researchers are responsible for any costs imposed by SURE to access the data. ALSWH Contact Person These data have a number of unique characteristics and a user must talk to an ALSWH staff member who is knowledgeable about the data as misunderstanding the data could easily occur. For example, the meaning of some 1 EoI #- Date Received- fields may not be clear and some information may not be included in the data. A new user may first be required to navigate around a mock up data file before accessing the true file. Projects will not be approved unless the ALSWH Publications Substudies and Analyses (PSA) Committee and the ALSWH Data Linkage Committee (DLC) are assured that the prospective user understands how to use these datasets appropriately. 2 EoI #- Date Received- The variables listed below are specific to each data set in each state. The lists of variables available are different across states. Please indicate the variable(s) requested for each state(s) and provide sufficient justification for their inclusion in your study. To prevent potential delay, it is strongly recommended applicants spend time to provide sufficient justification for all requested variables before submitting an application as the release of variables will not be considered unless sufficient justification has been provided. 1973-78 Cohort 1946-51 Cohort 1921-26 Cohort ☐ ☐ ☐ 1. CANCER REGISTRY DATA NSW Cancer Registry- Variable Checklist Variable ☐ Age at diagnosis (years) ☐ Date of diagnosis ☐ Cancer type ☐ ICD- 0-3 topography code ☐ ICD- 0-3 morphology code ☐ Best basis of diagnosis ☐ Degree of spread ☐ Date of death ☐ ICD- 0-3 cause of death Justification QLD Cancer Registry- Variable Checklist Variable ☐ Date of diagnosis ☐ Site of cancer ☐ Differentiation ☐ Morphology ☐ Basis of diagnosis ☐ Date of death ☐ ICD-O cause of death Justification For Breast Cancer: ☐ Tumour size ☐ Number of nodes ☐ Number of positive nodes ☐ Laterality 3 EoI #- Date Received- ☐ Nodal status complete ☐ Size complete For Melanoma: ☐ Thickness of lesion WA Cancer Registry- Variable Checklist Variable ☐ Date of death ☐ Cause of death ☐ ☐ Justification Pathology record sequence number Tumour site/topography code ☐ Tumour morphology ☐ Tumour behaviour ☐ Basis of diagnosis ☐ Tumour grade ☐ Date of diagnosis ☐ Postcode of residence at diagnosis ☐ Fatality flag ☐ WACR cancer type code ☐ Cause of death type ☐ Pathology ☐ Tumour 'status' code ☐ Date validity ☐ Reliability code for unusual code combinations VIC Cancer Registry- Variable Checklist Variable ☐ Justification Age at diagnosis Tumour Demographics ☐ Primary Site (ICDO-3) ☐ Primary Site (ICD-10) ☐ Morphology (ICDO-3) ☐ Behaviour 4 EoI #- Date Received- ☐ Grade ☐ Laterality ☐ Date of diagnosis ☐ Basis of diagnosis ☐ Size ☐ Melanoma level ☐ Melanoma thickness ☐ Nodes sampled ☐ Nodes positive SA Cancer Registry- Variable Checklist ☐ Date of death ☐ Cause of death ☐ Date of diagnosis ☐ Primary site (ICD-0-3) ☐ Morphology (ICD-0-3) ☐ Behaviour ☐ Investigations ☐ Staging Information ACT Cancer Registry- Variable Checklist ☐ Age of diagnosis (years) ☐ Date of diagnosis ☐ Cancer type ☐ Topography code (ICD-0-3) ☐ Morphology code (ICD-0-3) ☐ Best basis of diagnosis ☐ Degree of Spread ☐ Death of Date ☐ Cause of Death (ICD-0-3) 5 EoI #- Date Received- 2. PERINATAL DATA NSW Perinatal Data- Variable Checklist Variable ☐ Mother’s age ☐ Mother’s marital status ☐ Baby’s date of birth ☐ Date of LMP ☐ Previous pregnancy > 20 weeks gestation? ☐ Number of previous pregnancies ☐ Was last birth by caesarean section? ☐ Total number of previous caesarean section ☐ CVS/Amniocentesis ( <20 weeks) ☐ Cervical suture ☐ Antenatal care ☐ Duration or pregnancy (weeks) at first antenatal visit ☐ Smoking during pregnancy ☐ Maternal diabetes mellitus ☐ Gestational diabetes ☐ Chronic hypertension ☐ Pregnancy-induced hypertension ☐ ☐ ☐ ☐ ☐ ☐ ☐ Justification Pregnancy-induced hypertensionproteinuric Pregnancy-induced hypertension nonproteinuric Prelabour rupture of membranes (>24 hours) Labour onset Induction and augmentation of labour by other method Main indication for induction/augmentation of labour Pain relief: None ☐ Nitrous oxide ☐ IM narcotics ☐ Local to perineum ☐ Pudendal block ☐ Epidural/caudal block ☐ Spinal anaesthetic 6 EoI #- Date Received- ☐ General anaesthetic ☐ Other ☐ Analgesia for labour: Nil ☐ Nitrous oxide ☐ Systemic opioids ☐ Spinal ☐ Epidural/caudal ☐ Combined spinal epidural ☐ General anaesthetic ☐ Other ☐ Epidural anaesthetic ☐ Type of delivery ☐ Baby’s sex ☐ Plurality of birth ☐ Birth weight ☐ APGAR score (1 min) ☐ APGAR score ( 5 min) ☐ Birth order ☐ Onset or Augmentation of labour ☐ Estimated gestational age ☐ Induction/Augmentation by oxtocics ☐ Induction/Augmentation byPGs ☐ Induction/Augmentation by ARM ☐ Delivery QLD Perinatal Registry- Variable Checklist Variable ☐ Mother’s date of admission ☐ Mother’s marital status ☐ Mother’s age ☐ Previously pregnant indicator ☐ Total number of previous pregnancies ☐ Last menstrual period ☐ Number of antenatal visits ☐ Number of previous caesareans ☐ Number of previous pregnancies resulting in all live births Justification 7 EoI #- ☐ ☐ Date Received- Number of previous pregnancies resulting in all stillbirths Number of previous pregnancies resulting in all abortion/miscarriage/ectopic/hydatiform moles ☐ Antenatal care flag ☐ Nuchal translucency ultrasound ☐ Morphology ultrasound ☐ Chronicity ultrasound ☐ ☐ ☐ ☐ ☐ ☐ Average number of cigarettes smoked Average number of cigarettes smoked per day during the first 20 weeks of pregnancy Average number of cigarettes smoked per day after the first 20 weeks of pregnancy Smoke status for the whole pregnancy Cigarette smoking indicator during the first 20 weeks of pregnancy Cigarette smoking indicator after the first 20 weeks of pregnancy ☐ Gestation at first antenatal visit ☐ Mother’s medical condition (ICD-10) ☐ Flag for pre-existing diabetes (1=yes) ☐ Pregnancy complications (ICD-10) ☐ Flag for gestational diabetes (1=yes) ☐ Flag for gestational hypertension (1=yes) ☐ Neonatal morbidities (ICD-10) ☐ Congenital anomalies (ICD-10) Baby’s Birth Detail Record ☐ Birth plurality ☐ Birth order ☐ Onset of labour ☐ Primary reason for induction ☐ Methods of inducing labour or augmenting labour after spontaneous onset ☐ Anaesthesia flag ☐ Method of birth ☐ Reason for caesarean ☐ Birth weight ☐ Gestation age completed weeks ☐ Birth status of baby ☐ Baby’s sex 8 EoI #- Date Received- ☐ APGAR score at 1 minute ☐ APGAR score at 5 minutes ☐ Membranes rupture time WA Midwives Notification System- Variable Checklist Variable Justification Mother’s Details ☐ Marital status ☐ Height Pregnancy Details ☐ Previous pregnancies ☐ Previous pregnancy outcomes ☐ Previous caesarean section ☐ Caesarean last delivery ☐ Previous multiple birth ☐ Date of last menstrual period ☐ Smoking during pregnancy ☐ Complications of pregnancy ☐ Medical conditions ☐ Procedures/treatments Labour Details ☐ Onset of labour ☐ Augmentation ☐ Induction ☐ Analgesia (during labour) Delivery Details ☐ Anaesthesia (during delivery) ☐ Complications of labour and delivery Baby Details ☐ Date of birth ☐ Plurality ☐ Method of birth ☐ Gender ☐ Status of baby at birth ☐ Infant weight ☐ Length of baby (cms) 9 EoI #- Date Received- ☐ Head circumference ☐ APGAR score at 1 minute ☐ APGAR score at 5 minutes ☐ Estimated gestation ACT Perinatal Data Collection ☐ Mothers age ☐ Mother’s marital status ☐ Baby’s date of birth ☐ Date of LMP ☐ Previous pregnancies ☐ Number of previous pregnancies ☐ Was last birth by caesarean section? ☐ Total number of previous caesarean section ☐ Amniocentesis (<20 weeks) ☐ Amniocentesis (≥20 weeks) ☐ CVS ☐ Cervical suture ☐ Antenatal care providers ☐ Duration of pregnancy (weeks) at first antenatal visit ☐ Smoking during pregnancy ☐ Gestational diabetes ☐ Chronic hypertension ☐ Pregnancy-induced hypertension ☐ Prelabour rupture of membranes (>24 hours) ☐ Labour onset ☐ Type of Induction ☐ Type of Augmentation ☐ Analgesia for labour ☐ Anaesthesia for delivery ☐ Type of Delivery ☐ Method of Birth ☐ Baby’s sex ☐ Plurality of birth ☐ Birth order ☐ Gestational age 10 EoI #- Date Received- ☐ Birth weight ☐ APGAR score (1 min) ☐ APGAR score (5 min) 11 EoI #- Date Received- 3. ADMITTED PATIENTS DATA NSW Admitted Patients Data Variable ☐ Age ☐ Marital status ☐ Source of referral ☐ Date of admission ☐ Date of separation ☐ Length of stay ☐ Number of leave days ☐ Primary diagnosis ☐ Additional diagnosis ☐ External cause of injury or poisoning ☐ Activity when injured ☐ Place of occurrence ☐ Principal procedure ☐ Date of principal procedure ☐ Additional procedures ☐ Insurance status ☐ Health insurance on admission ☐ Episode start date ☐ Episode start time ☐ Episode end date ☐ Episode end time ☐ Clinical codeset ☐ Mode of separation ☐ Local Health District of hospital ☐ Days in a Designated Psychiatric Unit ☐ Payment status on separation Major Diagnosis Category for Australian Refined Diagnosis Related Group Australian Refined Diagnosis Related Group Australian Refined Diagnosis Related Group version ☐ ☐ ☐ Justification 12 EoI #- Date Received- ☐ Episode of care type ☐ Service related group ☐ Service related group version QLD Hospital Admitted Patients Data Variable ☐ Admission date ☐ Separation date ☐ Length of stay ☐ ☐ Justification Mode of separation (discharge status, grouped) Source of referral/transfer (admission source) ☐ Hospital insurance status ☐ Funding source ☐ Care type ☐ Elective patient status ☐ ICD-10AM Principal diagnosis ☐ ICD-10AM Other diagnosis- up to 39 per episode ☐ ICD-10AM External cause ☐ ICD-10AM Morphology code ☐ ☐ ICD-10AM Procedure code- up to 43 per episode Date of procedure(s)- up to 33 per episode WA Hospital Morbidity Data Patient Information ☐ Admission age ☐ Admission date ☐ Separation date ☐ Marital status ☐ Employment status ☐ Length of stay ☐ Source of referral: location ☐ Source of referral: professional Only available on data after July 2000 13 EoI #- ☐ ☐ Date Received- Source of referral: transport Source of referral (Available on data prior to July 2000) ☐ Admission status ☐ Insurance status ☐ Care type ☐ Total leave days ☐ Number of leave periods ☐ Days of hospital in the home care ☐ Days in ICU ☐ Hours CVS ☐ Mode of separation Diagnosis Codes ☐ Principal diagnosis ☐ Co-diagnosis ☐ Additional diagnoses Procedure Codes ☐ Principal procedure ☐ Additional procedures E-Codes ☐ External cause of injury ☐ Activity code ☐ Place of occurrence South Australia Separations (Hospital) ☐ Admission category ☐ Admission date ☐ Admission type ☐ Separation type ☐ Principal diagnosis ☐ Additional diagnoses ☐ Episode of care ☐ External cause ☐ Hospital insurance ☐ Transfer to hospital ☐ Transfer from hospital 14 EoI #- Date Received- ☐ Marital status ☐ Nature of separation ☐ Length of stay (days) ☐ Place of occurrence ☐ Procedure ☐ Referral further health care ☐ Separation date ☐ Source of referral ☐ Admission election ☐ Separation election ☐ Activity Category ☐ Date of Birth Category ACT Admitted Patient Care (APC) ☐ Age ☐ Marital Status ☐ Date and time of admission ☐ Date and time of separation ☐ Length of stay (LOS) ☐ Number of leave days ☐ Day stay flag ☐ Primary diagnosis ☐ Additional diagnoses ☐ External cause of injury or poisoning ☐ Activity when injured ☐ Place of occurrence ☐ First procedure ☐ First procedure date ☐ Other procedures ☐ Admitted to psychiatry ward ☐ Days in a designated psychiatric unit ☐ Insurance status ☐ Major Diagnosis Category (MDC) ☐ Australian Refined Diagnosis Related Group ☐ Service Related Group 15 EoI #- Date Received- ☐ Separation mode Office Use Only EOI No: Date EOI approved: Email address __ /__ /___ Dataset created by: Date dataset created: __ /__ /___ 16