Unit 8 Measurement powerpoint information ppt
advertisement

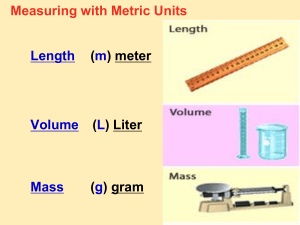
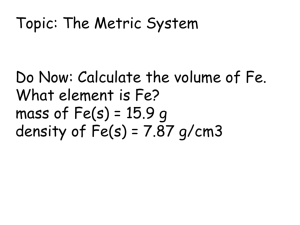
Measurement In Chemistry Measurement At the conclusion of our time together, you should be able to: 1. Explain the difference between the two types of observations 2. Give examples of 5 basic SI measuring units 3. Give examples of 3 different derived units from the basic SI units 4. Vary the amounts of the SI measuring units with 10 different prefixes and define each 5. Explain the difference between mass and weight 6. List and explain 5 different units for volume. Types of Observations and Measurements We make QUALITATIVE observations of reactions — changes in color and physical state. We also make QUANTITATIVE observations that involve MEASUREMENTS with numbers and units. Some Tools for Measurement Which tool(s) would you use to measure: A. temperature B. volume C. time D. weight Stating a Quantitative Measurement In every measurement there is a Number (Quantity) followed by a Unit from a measuring device Quantitative Measurements in Chemistry Must Include Units! Standards of Quantitative Measurement When we measure, we use a measuring tool to compare some dimension of an object to a standard. For example, at one time the standard for length was the king’s foot. What are some problems with this standard? SI Measurement Le Système International d‘Unités Adopted in 1960 by the General Conference on Weights and Measures. International Standards are kept in France. Among countries with nonmetric usage, the U.S. is the only country significantly holding out. The U.S. officially adopted SI in 1866. Liberia and Myanmar are changing over. Liberia Information from U.S. Metric Association Base SI Units Quantity Unit Symbol Length meter m Mass kilogram kg Temperature kelvin K Time second s Amount of Substance mole mol Luminous Intensity candela cd Electric Current ampere a Units Of Measurement You Must Know Use SI units — based on the metric system Length Mass meter, m kilogram, kg Volume Time Temperature liter, L seconds, s kelvin, K Unit for Length 1 Meter Note: Not Foot!! Metric Prefixes Kilo- means 1000 of that unit 1 kilometer (km) = 1000 meters (m) Centi- means 1/100 of that unit 1 meter (m) = 100 centimeters (cm) 1 dollar = 100 cents Milli- means 1/1000 of that unit 1 meter (m) = 1000 millimeters (mm) Metric Prefixes Metric Prefixes You Will Need to Know: Grand Master King Henry Died by Drinking Chocolate Milk Monday Night Metric Prefixes You Need to Know 103 103 meters = 1 km 1 meter (m) 1 dm (decimeter) 1 cm (centimeter) 1 mm (millimeter) 10 1 10-1 10-2 10-3 10 dm = 1 m 100 cm = 1 m 103 mm = 1 m 1 m (micrometer) 10-6 106 m = 1 m 1 nm (nanometer) 10-9 109 nm = 1 m 1 kilometer (km) Unit for Mass 1 Kilogram Note: Not Gram!! Unit for Weight 1 Newton 1 N = kg m/s2 A Weighty Problem On 9/23/99, $125,000,000 Mars Climate Orbiter entered Mars’ atmosphere 100 km lower than planned and was destroyed by heat. 1 lb = 1 N 1 lb = 4.45 N “This is going to be the cautionary tale that will be embedded into introduction to the metric system in elementary school, high school, and college science courses ‘till the end of time.” Mass and Weight Weight is force of the gravitational pull on an object. It would be different on the moon than it is on earth. Mass is a measure of the amount of matter in an object. Mass and weight are directly related as long as we remain on earth at the same elevation. That is, if one object has twice the mass of another, then its weight on earth would also be twice as large. However, if we take the same object to Denver, Colorado, the mass stays the same but the weight would be different. Why?? Mass vs. Weight Mass: Amount of Matter (grams, measured with a BALANCE) Weight: Force exerted by the mass, only present with gravity (pounds, measured with a Scale) Can you hear me now? Unit for Volume 1 Meter Cubed Note: A Derived Unit!! To Big, So Generally Use the Liter What is a Liter?? Length and Volume 1 m = 10 dm therefore 1 m3 = 103 dm3 1 dm = 10 cm therefore 1 dm3 = 103 cm3 1 L = 1 dm3 and 1 L = 103 mL so 1 L = 1 dm3 = 103 mL = 103 cm3 = 103 cc so This means that 1 milliliter (mL) is the same as 1 cubic centimeter (cc). These terms are often used interchangeably. Units for Volume m3 cm3 1 dm3 = 1 L dm3 L mL Liter 1 cm3 = 1 mL Other Derived SI Units Quantity Unit Symbol Volume cubic meter m3 Density kilograms per cubic meter kg/m3 Speed meter per second m/s Newton kg m/ s2 N Energy Joule (kg m2/s2) J Pressure Pascal (kg/ms2) Pa Units for Energy Joule calorie J 1 cal = 4.184 J 1 cal = quantity of heat needed to raise the temperature of 1 g of water by 1 oC. 1 kcal = 1000 cal Measurement Let’s see if you can: 1. Explain the difference between the two types of observations 2. Give examples of 5 basic SI measuring units 3. Give examples of 3 different derived units from the basic SI units 4. Vary the amounts of the SI measuring units with 10 different prefixes and define each 5. Explain the difference between mass and weight 6. List and explain 5 different units for volume. Learning Check Match L) length M) mass V) volume M A. ____ A bag of tomatoes is 4.6 kg. L B. ____ A person is 2.0 m tall. M C. ____ A medication contains 0.50 g Aspirin. V D. ____ A bottle contains 1.5 L of water. Learning Check What are some U.S. units that are used to measure each of the following? A. length B. volume C. weight D. temperature Which Metric Prefix is Generally Used? Learning Check 1. 1000 m = 1 ___ a) mm b) km c) dm 2. 0.001 g = 1 ___ a) mg b) kg c) dg 3. 0.1 L = 1 ___ a) mL b) cL c) dL 4. 0.01 m = 1 ___ a) mm b) cm c) dm Learning Check Select the unit you would use to measure 1. Your height a) millimeters b) meters c) kilometers 2. Your mass a) milligrams b) grams c) kilograms 3. The distance between two cities a) millimeters b) meters c) kilometers 4. The width of an artery a) millimeters b) meters c) kilometers