States of Matter Webquest - Tri
advertisement
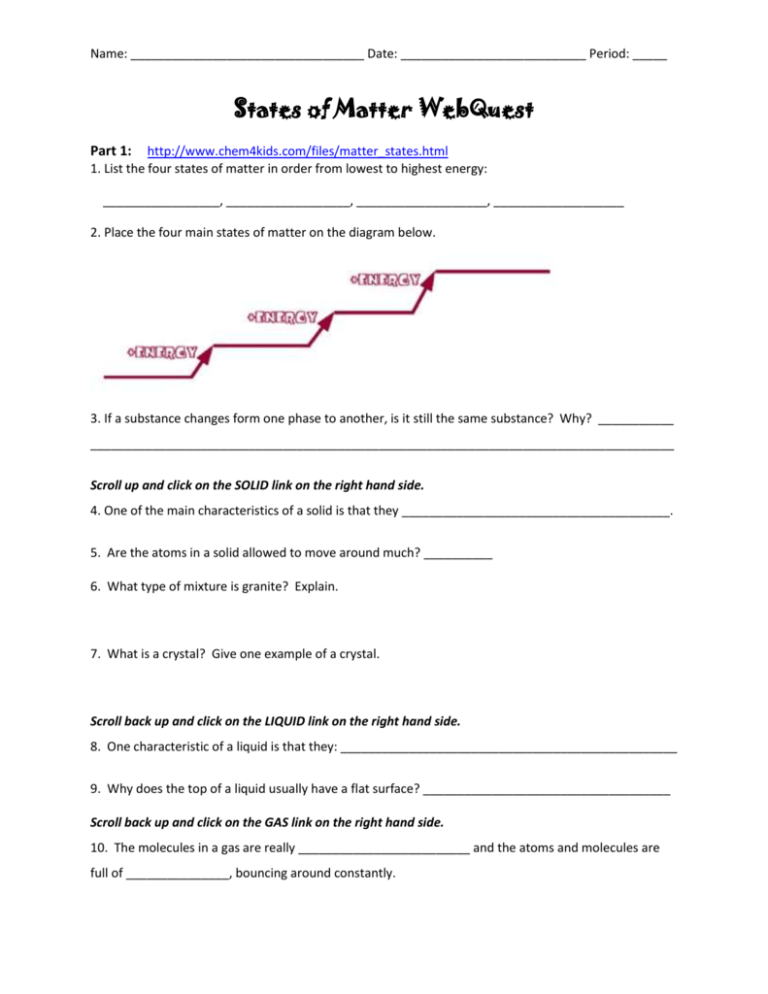
Name: __________________________________ Date: ___________________________ Period: _____ States of Matter WebQuest Part 1: http://www.chem4kids.com/files/matter_states.html 1. List the four states of matter in order from lowest to highest energy: _________________, __________________, ___________________, ___________________ 2. Place the four main states of matter on the diagram below. 3. If a substance changes form one phase to another, is it still the same substance? Why? ___________ _____________________________________________________________________________________ Scroll up and click on the SOLID link on the right hand side. 4. One of the main characteristics of a solid is that they _______________________________________. 5. Are the atoms in a solid allowed to move around much? __________ 6. What type of mixture is granite? Explain. 7. What is a crystal? Give one example of a crystal. Scroll back up and click on the LIQUID link on the right hand side. 8. One characteristic of a liquid is that they: _________________________________________________ 9. Why does the top of a liquid usually have a flat surface? ____________________________________ Scroll back up and click on the GAS link on the right hand side. 10. The molecules in a gas are really _________________________ and the atoms and molecules are full of _______________, bouncing around constantly. Name: __________________________________ Date: ___________________________ Period: _____ 11. One of the physical characteristics is that a gas can ________________________________________. Scroll back up and click on PHASE CHANGE 1 on the right hand side. 12. Fill in the missing piece of information. CHEMISTRY TERM PHASE CHANGE __________________________ Solid to Liquid Freezing ____________________ ___________________________ Liquid to Gas Condensation ____________________ ___________________________ Solid to Gas Deposition ____________________ 13. Atoms in a liquid have __________ energy than atoms in a solid, so the easiest way to change a solid to a liquid is to add __________. When changing from a solid to a liquid, there is a magic temperature for each substance called the ________________________________________. Part 2: http://www.mansfieldct.org/schools/mms/staff/hand/atomsheat.htm 14. Explain what happens when heat is added to a substance. Be specific. 15. What happens to the mass of the objects as the temperature of the substance changes? Part 3: www.classzone.com/books/ml_science_share/vis_sim/mem05_pg101_kintheory/mem05_pg101_kinth eory.html 16. As the kinetic energy is adjusted, explain what happens to the temperature. Is it a direct or inverse relationship? 17. When the mass is adjusted, the temperature stays the same. Describe how the speed of the molecules changes. Part 4: http://www.vtaide.com/png/matter.htm Complete this matter quiz. 18. How did you do? (Circle one) 1 (I had to fix a lot of my answers) 2 (had to fix some of my answers) 3 (I only had to fix 1 or 2) Name: __________________________________ Date: ___________________________ Period: _____ Part 5: http://www.chem.purdue.edu/gchelp/atoms/states.html 19. Use the chart to identify the state of matter described by the following. Many of these have more than one answer! (Use S, L or G in the spaces.) _______ not easily compressible _______rigid – particles locked into place _______flows easily _______ compressible _______ lots of free space between particles _______ does not flow easily _______ assumes the shape of the part of the container which it occupies _______ particles can move past one another _______ retains a fixed volume and shape _______assumes the shape and volume of its container _______ little free space between particles Part 6: http://www.quia.com/quiz/303980.html Take this quiz to test your knowledge on physical and chemical changes. 20. How did you do?