Handout
advertisement

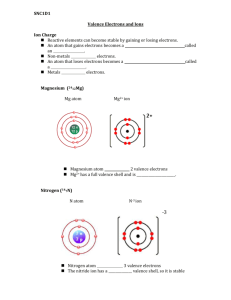
Ions (Cations & Anions) When atoms gain or lose electrons, they acquire either a positive or negative charge. 1. What is the charge on an electron? 2. When an atom loses an electron, what charge will it have? 3. When an atom gains an electron, what charge will it have? 4. Ion = 5. Positively and negatively charged atoms have special names. a. Cation: b. Anion: 6. So – if you have an atom of chlorine (Cl), how do you make it into an anion? 7. Sometimes atoms can gain or lose more than one electron. Let’s work through the following. a. How many valence electrons do alkaline earth metals have? b. They will have the tendency to give these both away to become more stable. What do you think is the symbol for a calcium (Ca) atom (an alkaline earth metal) that has given away both of its electrons? c. Apply this to the halogens – pick a halogen element, figure out if it will want to gain or lose electrons, how many and then write the symbol for the ion. 8. Nitrogen (N) has an atomic number of 7 meaning it has 7 protons. A neutral atom of Nitrogen has the same number of electrons – 7. Of those 7 electrons, 5 are valence electrons. a. Will it have a tendency to lose or gain electrons? How many? b. Write the ion symbol showing the charge. c. This question can be asked a different way – If an ion of nitrogen has 7 protons and 10 electrons, what is the ion symbol? How do you figure this out? 9. Review the description of an isotope from the Atomic Theory unit. Distinguish between isotopes and ions. Use the diagrams as support. Notice the difference in how isotope symbols are written versus how ion symbols are written. Cl Chlorine Atom ClChlorine Ion 7 valence e- 8 valence e- Work through the following using the concepts we’ve learned so far. 1. If a carbon atom (C) gains 4 electrons… a. What will its charge be? Write the ion symbol. b. Is it an anion or cation? Why? 2. Will a fluorine atom (F)… a. gain or lose electrons? How many? b. Write the ion symbol and identify as a cation or anion. 3. Use the periodic table. a. What is the atomic number for lithium (Li)? What does this number represent? b. How many electrons does a neutral atom of Lithium have? c. How many valence electrons does lithium have? d. Will it gain or lose electrons? e. Write the ion symbol. f. Is it an anion or cation? 4. Use the periodic table. a. Identify the element whose ion has 4 protons and 2 electrons. b. Write the ion symbol. c. How many electrons does a neutral atom of this element have?