Periodic Table Review_ CC
advertisement

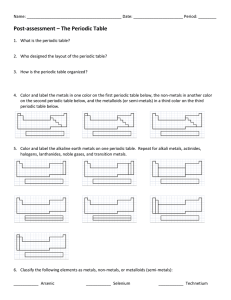
Name_____________________________________ TEST DATE__________________________________ Per_________ Periodic Table Assessment: REVIEW 1. 2. On the periodic table above, label the group names. For each group, identify the number of valence electrons all elements in that group have, whether they will gain or lose electrons to achieve the Octet Rule and what will be the charge of the ion that results. Group # 1A Group Name Alkali Metals # of Valence Electrons Gain or lose electrons? How many? 1 Lose 1 electron 2A 3A 4A 5A 6A 7A 8A “B” Groups (I-VIII) Not numbered 3. Inner transition metals What is the difference between a period and a group? Ion Charge +1 4. Who created the first periodic table? ____________________________________________ What was so fascinating about this first periodic table? 5. Use your notes and/or periodictable.com to fill in properties of metals/non-metals/semi-metals. Metals Semi-Metals Non-metals 6. 7. 8. Where are the metals found on the periodic table?___________________________________________ Where are the non-metals found on the periodic table?________________________________________ Where are semi-metals found on the periodic table? __________________________________________ 9. Use a periodic table to find the following information about each element. Calcium (Ca) Oxygen (O) Atomic number_________ Atomic mass___________ # of protons___________ # of neutrons__________ Total # of electrons in an atom with no charge_______ # of valence electrons______ Ion it will form ________ Metal, Non-metal or Semi-metal_____________ Atomic number_________ Atomic mass___________ # of protons___________ # of neutrons__________ Total # of electrons in an atom with no charge_______ # of valence electrons______ Ion it will form ________ Metal, Non-metal or Semi-metal______________ Draw an electron diagram for this atom: Draw an electron diagram for this atom: Draw an electron diagram for its ion: Draw an electron diagram for its ion: