Spectrum
advertisement

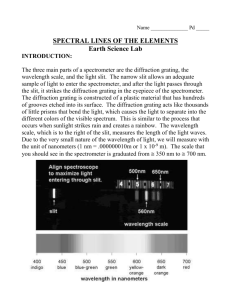
Spectrum Objective: To observe the emission spectrum of hydrogen To measure the wavelength of the emission lines for hydrogen spectrum To compare the measured energies of the photons with Bohr’s prediction Equipment: Spectrum tube power supply Transmission diffraction grating Helium, Mercury, and Neon discharge tube Wooden spectrometer apparatus Theoretical Background: Light is an electromagnetic wave. The wavelength of visible light ranges from about 400 nm for blue-violet to 700 nm for red. It is possible to separate the light into its constituent components. The pattern of resulting colors after separation is called a spectrum. There are three different types of spectra. These are continuous spectra, bright-line (emission), and dark-line (absorption) spectra. A hot dense gas object will produce a continuous spectrum whereas a hot transparent gas will produce an emission spectrum. A continuous band of colors are visible in a continuous spectrum. In an emission spectrum, a series of bright lines are visible against a dark background. In contrast, a series of dark lines are visible against a continuous spectral background for a absorption spectrum. The dark lines seen in the absorption spectrum represents the wavelengths of the light which are absent. By observing the spectrum, it is possible to determine the properties of the light source. This is the method which is used to determine the features of the distant stars and galaxies as well as objects in our solar system. . When an electron undergoes a transition from a higher state with higher energy to a lower state with lower energy, then the atom will emit a photon of energy E E i E f 1 (4) where E hf or E h c (5) In this laboratory, a transmission diffraction grating will be used to produce bright-line spectra from hydrogen gas-discharge tubes. A transmission diffraction grating is a just a piece of material having a large number of equally separated slits. Typical distance between the slits – grating spacing – is on the order of the wavelength of the light and varies from about 500 nm to 2000 nm . Procedure: 1. Place the grating in the grating slot of the apparatus. 2. Measure and record the distance from the grating to the slit, L , in data table. 3. Record the number of lines per unit length, n, for the transmission grating. 4. Use extreme caution when using spectrum-tube power supply. Do not touch the supply electrodes while the supply is turned on. Replace the discharge tubes only when the power supply is turned off. 5. Place the helium, mercury, or neon discharge tube in the tube holder of the spectrumtube power supply. 6. Align the light source such that the slit, as seen through the grating, is as brightest as possible as shown below. meter stick Balmer line eye grating Hydrogen gas L 7. Look straight through the grating. 8. A first-order Balmer lines should be visible on the left and the right sides of the discharge tube. 9. Measure and record the position of Balmer lines on the left, x left , and the right, x right , of the slit in Data Table 1. 10. Replace the hydrogen source with another source. 11. Measure and record the location of the strongest visible line. 2 Data Sheet: Data Table 1: Hydrogen Spectrum Distance from the slit to the grating, L = ______________ Number of grating per unit length = ___________________ Data Table 1: Helium Spectrum Color xhigh xlow xaverage xlow xaverage xlow xaverage Data Table 2: Mercury Spectrum Color xhigh Data Table 3: Neon Spectrum Color xhigh Calculations: From the number of lines per unit length on the grating, calculate the distance between the slits on the grating. Show calculations for the wavelength and the frequency of each line. Using the frequency, calculate the experimental value of the energy of the emitted photon corresponding to each line. Determine the theoretical value of the emitted photon energy. 3 Calculate the percent error between the expected and the experimental photon energies. Determine the wavelength of the unknown source. Using the spectrum chart, identify the element in the second discharge tube. Results: Results Table 1: Helium Spectrum wavelength Color average Results Table 2: Mercury Spectrum wavelength Color average Results Table 1: Neon Spectrum wavelength Color average frequency f Eexperimental Etheoretical frequency f Eexperimental Etheoretical frequency f Eexperimental Etheoretical 4