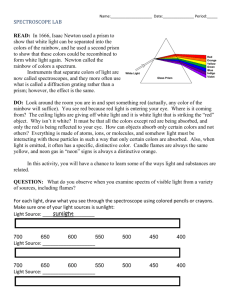
Spectra Activity
Answer Key
Activity 1
Purpose: to investigate how the color of light help us understand the matter
Material: flashlight and packet of gel color filters
Procedure: it is important to make careful observations during each step of the investigation.
1. While the overhead light is on, shine a flashlight on a white paper. Explain why the paper looks
brighter when a flashlight is shining on it. (Possible Answer: The addition of the overhead light
and the flashlight made the paper look brighter. There is more light reflecting back to my eyes
when both light sources are on. This is evidence that brighter light is made when light from
various sources is added together.)
2. Predict what color of light you will see on the paper when you aim three flashlights covered
with red, green and blue gel filter so that their circles are overlapping.
Prediction:____________________________
Join with other groups to share flashlights and try it. What color do you see? _________
Explain why you saw the color you did when all three colors of light were overlapping.
(Possible Answer: White. Red, blue and green are the primary colors of light. When all the colors
of light are added together, the light is the brightest it can be and reflects white light to my eyes.
This is evidence that white light is composed of red, green and blue.)
3. Now what do you see on the paper when a red filter is placed on the flashlight?
__________Why do you see this color? (Possible Answer: The white paper has a red circle of
light on it. There is red light reflecting back to my eyes. This is evidence that some parts of the
white light from the flashlight are absorbed by the red filter. The colors that must be absorbed
are the blue and the green since we know the light from the flashlight is white.)
4. Team up with another group. Try mixing the light of red and green colors of gel filter over two
flashlights. Is the new color you see is brighter than the original colors you mixed? Explain why
this happens. (Possible Answer: Yes, the color from two flashlights shining on the same spot will
be brighter no matter what 2 color filters are chosen. When more light is added together, the
brighter the light will be. Also, the combined light is yellow. This shows that combining light is
different than combining pigments.)
(C) Copyright 2014 - all rights reserved www.cpalms.org
Activity 2
Purpose: to investigate color in light
Material: flashlight, packet of gel color filters and a piece of C-Spectra or a spectroscope for each
student.
Procedure:
1. Look through the C-spectra toward a white light of the flashlight. Make a list of the colors that
you see. (Possible Answer: Red, orange, yellow, green, blue, indigo and violet – ROY G BIV)
___________________________________________________________________
2. Predict what you will see if you cover the flashlight with a yellow filter and then look toward the
yellow light using the piece of C-Spectra. Predicted colors:_____________________
Now do it and observe carefully. What colors do you see?______________________________
(Possible Answer: dull red, orange and yellow.)
3. What happens to the other colors of light when it goes through a yellow colored filter?
(Possible Answer: When the yellow filter is added, the blue and violet colors disappear. These
colors are absorbed by the yellow filter. The yellow and orange light is transmitted without any
change. It is important for to students to know that the missing colors were absorbed by the
filter.)
(C) Copyright 2014 - all rights reserved www.cpalms.org
Activity 3
Purpose: to investigate other filters of light
Material: C-spectra, colored pencils, markers or crayons.
The continuous spectrum below occurs when all light wavelengths are displayed.
Image from http://commons.wikimedia.org/wiki/File:Spectral_lines_continous.png
Procedure: After the gas tube is connected to the power supply, look at the tube through the Cspectra film. As you look at the light transmitted from each gas, draw each color line that you see in
the box. Instead of coloring the black you see, just leave it white.
This pattern of light is the emission spectrum of the gas you are viewing.
1. Emission Spectrum of Gas 1 :_H- Hydrogen_
700 nm
Red
600nm
Orange Yellow
500 nm
Green
Blue
400 nm
Indigo
Wavelength
Violet
Color
2. Emission Spectrum of Gas 2:__He - Helium__
700 nm
Red
600nm
500 nm
Orange Yellow Green Blue
400 nm
Indigo
Violet
Wavelength
Color
400 nm
Violet
Wavelength
Color
400 nm
Indigo
Violet
Wavelength
Color
3. Emission Spectrum of Gas 3:___Ne- Neon_
700 nm
Red
600nm
500 nm
Orange Yellow Green Blue
Indigo
4. Emission Spectrum of Gas 4:__Hg - Mercury__
700 nm
Red
600nm
500 nm
Orange Yellow Green Blue
(C) Copyright 2014 - all rights reserved www.cpalms.org
Answers: Sodium is not available in a gas tube. A flame test will reveal the sodium emission spectrum.
Images of spectra are available from the http://astronomy.nju.edu.cn/~lixd/GA/AT4/AT404/HTML/AT40401.htm
Questions:
1. What did you learn about gases when you looked at them through the C-spectra film?
(Possible answer: Each gas showed different colors. Each gas was unique and had a unique
fingerprint as shown in its emission spectrum.)
2. Could you tell what an unknown gas is if you know its color pattern? After observing the
emission spectra of the gases, name the gas whose emission spectrum is shown below.
(Answer: hydrogen)
Violet Blue
Green
Red
Image from http://commons.wikimedia.org/wiki/File:Visible_spectrum_of_hydrogen.jpg
3. What do you know about the property of elements that could explain your observations?
(Possible Answer: Each element on the periodic tables has properties that are unique to that
element. Each element has different number of protons and electrons which make the element
different from any other element.)
(C) Copyright 2014 - all rights reserved www.cpalms.org
Spectra Activity
Activity 1
Purpose: to investigate how the color of light help us understand the matter
Material: flashlight and packet of gel color filters
Procedure: it is important to make careful observations during each step of the investigation.
1. While the overhead light is on, shine a flashlight on a white paper. Explain why the paper looks
brighter when a flashlight is shining on it.
2. Predict what color of light you will see on the paper when you aim three flashlights covered
with red, green and blue gel filter so that their circles are overlapping.
Prediction:____________________________
3. Join with other groups to share flashlights and try it. What color do you see? _________
Explain why you saw the color you did when all three colors of light were overlapping.
4. Now what do you see on the paper when a red filter is placed on the flashlight?
__________Why do you see this color?
5. Team up with another group. Try mixing the light of red and green colors of gel filter over two
flashlights. Is the new color you see is brighter than the original colors you mixed? Explain why
this happens.
Activity 2
Purpose: to investigate color in light
Material: flashlight, packet of gel color filters and a piece of C-Spectra or a spectroscope for each
student.
Procedure:
1. Look through the C-spectra toward a white light of the flashlight. Make a list of the colors that
you see. ___________________________________________________________________
2. Predict what you will see if you cover the flashlight with a yellow filter and then look toward the
yellow light using the piece of C-Spectra. Predicted colors:_____________________
Now do it and observe carefully. What colors do you see?______________________________
3. What happens to light when it goes through a colored filter?
(C) Copyright 2014 - all rights reserved www.cpalms.org
Activity 3
Purpose: to investigate other filters of light
Material: C-spectra, colored pencils, markers or crayons.
The continuous spectrum below occurs when all light wavelengths are displayed.
Image from http://commons.wikimedia.org/wiki/File:Spectral_lines_continous.png
Procedure: After the gas tube is connected to the power supply, look at the tube through the Cspectra film. As you look at the light transmitted from each gas, draw each color that you see in the
box. Instead of coloring the black you see, just leave it white.
This pattern of light is the emission spectrum of the gas you are viewing.
1. Emission Spectrum of Gas 1 :________________________________________
700 nm
Red
600nm
Orange Yellow
500 nm
Green
Blue
400 nm
Indigo
Wavelength
Violet
Color
2. Emission Spectrum of Gas 2:________________________________________
700 nm
Red
600nm
500 nm
Orange Yellow Green Blue
400 nm
Indigo
Violet
Wavelength
Color
3. Emission Spectrum of Gas 3:________________________________________
700 nm
Red
600nm
500 nm
Orange Yellow Green Blue
400 nm
Indigo
Violet
Wavelength
Color
4. Emission Spectrum of Gas 4:________________________________________
700 nm
Red
600nm
500 nm
Orange Yellow Green Blue
(C) Copyright 2014 - all rights reserved www.cpalms.org
Indigo
400 nm
Violet
Wavelength
Color
Questions:
1. What did you learn about gases when you looked at them through the C-spectra film?
____________________________________________________________________
____________________________________________________________________
2. Could you tell what an unknown gas is if you know its color pattern? After observing the
emission spectra of the gases, name the gas whose emission spectrum is shown below.
Violet Blue
Green
Red
___________
Image from http://commons.wikimedia.org/wiki/File:Visible_spectrum_of_hydrogen.jpg
3. What do you know about the property of elements that could explain your observations?
____________________________________________________________________
(C) Copyright 2014 - all rights reserved www.cpalms.org