CaCl2 transfections
advertisement

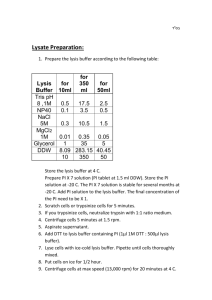
CaCl2 transfection of cells in culture 1. Plate cells and let them adhere for 6h (Or O.N). The cells should reach 70% density. 2. In eppendorf tubes mix transfection solution without 2xHBS: For a 10cm petri dish: 500μl DDW + plasmids (0.5μg OT/OF, 0.05μg β-Gal) 60μl [2M] CaCl2 500μl 2xHBS buffer Total volume: 1ml For a 6-well plate: 100μl DDW + plasmids (0.5μg OT/OF, 0.05μg β-Gal) 12μl [2M] CaCl2 100μl 2xHBS buffer Total volume: 200μl/ per well For a 24-well plate: 50μl DDW + plasmids (0.5μg OT/OF, 0.05μg β-Gal) 6μl [2M] CaCl2 50μl 2xHBS buffer Total volume: 100μl/ per well 3. Dived the transfection mix into 2 eppendorf tubes: #1 Add 0.5ug empty GFP plasmid #2 Add 0.5ug β-catenin plasmid 4. Add the 2x HBS drop by drop and vortex. 5. Incubate at R.T for 20-30 min. 6. Vortex and add the transfection solution to the plated cells drop by drop into the growth medium. 7. Incubate for 24h at 37C. 8. Change to a fresh medium containing activation protein. 9. Incubate for 24h at 37C. 10. Discard medium. 11. Wash 1xPBS. 12. Discard PBS and add 100μl lysis buffer + protease inhibitor. 13. Incubate 10min on ice. 14. Transfer the lysate to a fresh eppendorf tube. 15. Incubate 10min on ice. 16. Centrifuge for 15min, 13,000 rpm at 4C. 17. Perform Luciferase and β-Gal assays. 18. Luciferase assay: Add 50μl of luciferin buffer to 10μl of lysate supernatant and read immediately. 19. β-Gal assay: In a 96-well plate- Put 10μl of lysate supernatant, add 149μl ONPG buffer. Incubate at 37C for a few minutes or at R.T until color is develop. Read at 420nm. Luciferase assay 1. Luciferin: 100mg in 3.57ml 10mM Tris-Acetate (pH 7.8), 100μl aliquots at 20C. 2. ATP: 200mM in ??? DDW, 150μl aliquots 3. Luciferase buffer: 100mM Tris-Acetate (pH 7.8) 100mM MgAc 1mM EDTA (Tris-Acetate: 1M Tris adjusted to pH 7.8 with Acetic acid) For 500ml: 50ml Tris 1M 5ml MgAc 1M 1ml EDTA 0.5M 444ml DDW 100μl luciferin + 150μl ATP in 13.5ml luciferase buffer → 50μl per reaction For the reading: Add 50μl of luciferin buffer to 10μl of lysate supernatant and read immediately. β-Gal assay For each reaction: 30μl ONPG 1.5μl MgCl2 x100 115μl PBSx1 Total volume: 149μl In a 96-well plate: 10μl protein extract (lysate) 149μl ONPG mix Incubate at 37C for a few minutes or at R.T until color is develop. Read at 420nm. MgCl2 x100: 500μl MgCl2 [1M] 1.54ml β-MER 2.96ml DDW Total volume: 5ml ONPG: 4mg ONPG in 1ml PBSx1