UNIT 3 Structure and function of saccharides and carbohydrates
advertisement

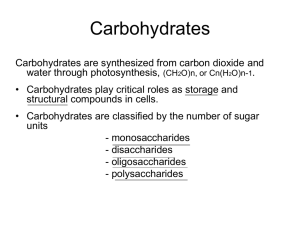
PRT3402- Agricultural Biochemistry PJJ UPM / UPMET UNIT 3 Structure and function of saccharides and carbohydrates Introduction to Unit Saccharides and carbohydrates are the most abundant class of biological molecules. Plants are the main producers of carbohydrates via photosynthesis. They have many special role in living cells whivh include energy provision when oxidized to drive metabolic processes. They act as energy storage molecules as as markers for cell recognition. In this unit you will learn about the basic components of carbohydrates which are saccharides. Starting with the structure of monosaccharides the build-up into oligosaccharides, polysaccharides and other polymers sucha starch, chitin and cellulose will be elucidated. Learning Outcomes At the end of this unit the students will be able to: 1. Recognise the basic chemistry and structure of simple sugars or saccharides and the existence of stereoisomers 2. Describe the formation of more complex structures of carbohydrates derived from monosaccharides. 3. Describe the various structure of and function of biologically important carbohydrate polymers. 33 PRT3402- Agricultural Biochemistry PJJ UPM / UPMET TOPIC 1: STRUCTURE OF SACCHARIDES, STEREOISOMERS, OLIGOSACCHARIDES AND THEIR DERIVATIVES Main Points 1.1 Carbohydrates can be classified as monosaccharides, oligosaccharides and poly saccharides. The term saccharides are derived from the Latin ‘sakcharon’ meaning sugar. Another name for monosaccharides is ‘simple sugars’. It contain between 3-7 C atoms and consists of a single polyhydroxy aldehyde (aldoses) or ketone unit (ketoses). Depending on the number of carbon atoms, different names are derived: No. of Carbon Name of monosaccharides 3 triose 4 tetrose 5 pentose 6 hexose 7 heptose 8 octose Monosaccarides made of aldehydes and ketones are called aldoses and ketoses respectively. Meanwhile monosaccharides that form five member rings and six member rings are called furanoses and pyranoses respectively. 1.2 Triose is the simplest 3 C monosaccharide and can exist as aldehyde (aldose) or ketones (ketose). Glyceraldehyde and dihydroxyacetone has the same atomic composition but differ only in the position of hydrogens and double bonds. 34 PRT3402- Agricultural Biochemistry 1.3 PJJ UPM / UPMET Other common monosaccharides include: Glucose (6C), Mannose (6C), Fructose (6C), Ribose (5C), Galactose (6C) and Erythrose (4C). Most abundance form of monosaccharide is D-glucose (6-Carbon). 1.4 Stereoisomers are compounds with the same molecular formula but different spatial arrangements of their atoms. The 3D arrangement of atoms around the carbon atom is such that if four groups are attached to it, they can be arranged in two different ways. Such C is described as asymmetric or chiral. The two molecules with different 3D arrangement are mirror images of each other. Mirror images of each other are called enantiomers. Two sugars that differ in configuration at only one chiral center are called epimers for e.g Dglucose and D-mannose are epimers. 1.5 Molecule with n chiral centers can have: 2n stereoisomers Glyceraldehyde = 1 chiral center 21 = 2 streoisomers By convention, one is a D isomer, the other L isomer. 35 PRT3402- Agricultural Biochemistry PJJ UPM / UPMET CHO CHO H C OH HO C CH2OH CH2OH D-glyceraldehyde L-glyceraldehyde CHO H C CHO OH HO C CH2OH D-glyceraldehyde 1.6 H H CH2OH L-glyceraldehyde For 6 carbon aldoses it will have 4 chiral centres. Thus: 24 = 16 streoisomers which is 8 D isomers and 8 L isomers. O H O C H – C – OH HO – C – H H – C – OH H – C – OH CH2OH C HO – C – H H – C – OH HO – C – H HO – C – H CH2OH D-glucose 1.7 H L-glucose The following structures depict the relationship of D-aldoses and their stereoisomers. 36 PRT3402- Agricultural Biochemistry PJJ UPM / UPMET 37 PRT3402- Agricultural Biochemistry 1.8 PJJ UPM / UPMET When sugars cyclize, they form furanose or pyranose structures. In aqueous solution, aldotetroses (4C) and other monosaccharides with 5 or more C atoms occur mostly as cyclic structures. For example C1 aldehyde and C5 1.9 -OH react to form a 6C ring pyranose structure. Cyclization of glucose produces a new asymmetric center at C1, e.g α-glucose (-OH below the ring) and β-glucose (-OH above the ring). A pair of stereoisomers that differ in configurations around C-1 are called anomers. Hence C-1 carbon is called anomeric carbon whilst α-glucose and β-glucose are anomers. Glucose (cyclic form) 1.10 Many of the monosaccharides plays very important functions in biological metabolism as indicated below: Number of Carbon Triose (3 C) Type of Monosaccharide Glyceraldehyde Dihydroxyacetone 38 Role/Function The 3-phosphate intermediate in glycolysis The 1-phosphate intermediate in PRT3402- Agricultural Biochemistry Pentoses (5 C): PJJ UPM / UPMET D-Arabinose L-Arabinose D-Ribose 2-D-Deoxyribose Hexoses (6 C) D-Glucose D-Galactose D-Mannose D-Fructose Heptulose (7 C) D-Sedoheptulose glycolysis Plant glycosides (defence), cellwalls Contituents of cell walls, plant glycoprotein Constituents of Deoxyribonucleic acid (DNA) Energy source Milk (part of lactose) Polysaccharides structures Intermediate in glycolysis Intermediate in Calvin cycle in photosynthesis 1.11. Monosaccharides can be added with other chemical groups which form other derivates of importance. Some of these derivatives include sugar phosphates, sugar acids, sugar alcohols, amino sugars and deoxy sugars. 1.12. Sugar phosphates are made by esterifying a phosphate group to one of the hydroxyls. The phosphate group gives molecules a negative charge at neutral pH. Some examples include glyceraldehyde phosphate, glucose-1-phosphate, glucose-6-phosphate and fructose-6-phosphate. Many of these sugar esters are important metabolic intermediates in the generation of ATP (adenosine triphosphate). These are shown below. 39 PRT3402- Agricultural Biochemistry PJJ UPM / UPMET 1.13. Sugar acids are monosaccharides with carboxyl groups. These include gluconic acid, glucuronic acid and ascorbic acid (Vitamin C). Glucuronic acid can be found in fermented drink. Some structures of these sugar acids are shown below. 40 PRT3402- Agricultural Biochemistry PJJ UPM / UPMET 1.14. As the name suggests sugar alcohols contain alcohol groups when the carbonyl part of the sugar is reduced. This group of sugars are known as alditols and include erythritol, D-mannitol and D-glucitol (or sorbitol). Mannitol occurs naturally in pineapples, olives, asparagus, sweet potatoes and carrots whereas sorbitol is found naturally in fruits and vegetables, xylitol is found naturally in fruit, vegetables, and cereals and Erythritol is found in fruit and fermented food and also tasted as sweet as table sugar. For this reason mannitol, sorbitol, xylitol are used to sweeten sugarless chewing gum and mints.The structures of these alditols are shown below. 41 PRT3402- Agricultural Biochemistry PJJ UPM / UPMET 1.15. Amino sugars are made by replacing hydroxyl of a sugar with an amine group. Some examples areβ –D-glucosamine and D-galactosamine. β–D-Nacetylglucosamine, muramic acid and N-acetylmuramic acid (sialic acid) are some derivatives of the amino sugars. Glucosamine is part of the structure of the polysaccharides chitosan and chitin, which compose the exoskeletons of crustaceans. Glucosamine is also used for the treatment of osteoarthritis. Galactosamine is a hepatotoxic or liver-damaging agent. These amino sugars and their derivatives are shown below. 42 PRT3402- Agricultural Biochemistry PJJ UPM / UPMET 1.16. In deoxy sugars, one of the OH groups is replaced with hydrogen. Deoxy sugars are constituents of DNA – deoxyribonucleic acid (See Unit 5). 1.17. Oligo in Greek means “few”, hence oligosaccharides consists of two to ten simple sugars. Oligosaccharides and polysaccharides are formed when monosaccharides are joined through glycosidic bonds. The simplest oligosaccharides, disaccharides include sucrose and lactose. Other 43 PRT3402- Agricultural Biochemistry PJJ UPM / UPMET disaccharides are trehalose, maltose, gentiobiose and cellobiose. Disaccharides are quite common whilst trisaccharides are frequently found. Oligosaccharides with four or six sugars are usually bound to other molecules. Maltose and trehalose consists of 2 glucose units while sucrose consists of glucose and fructose. 1.18. Maltose is a disaccharide of glucose units joined by -1,4 linkage while trehalose is a disaccharide made by joining two glucose units together via (1->1) bonds. Sucrose is a disaccharide, composed of a glucose joined through carbon 1 in -linkage to carbon 2 of fructose. Sucrose is a common table sugar. 1.19. Maltose is form from the glycosidic linkage between the C1 hydroxyl of one glucose and the C4 hydroxyl of another glucose. 44 PRT3402- Agricultural Biochemistry PJJ UPM / UPMET 1.20. Other common disaccharides include: Lactose -milk sugar composed of galactose & glucose with β(14) linkages and Cellobiose which is the product of cellulose breakdown. The β(14) glycosidic linkage is a zig-zag between 2 glucoses where one is actually flipped over the other. 1.21. Features that distinguish disaccharides from each other include: a). The two specific sugar monomers may be of the same kind or they may be different). b). The carbons involved in the linkage (the most common linkages are 1→1, 1→2 (as in sucrose), 1 → 4 (as in lactose, maltose and cellobiose). c) The order of monomeric units, if they are of different types. d). The anomeric configuration of the hydroxyl group on carbon 1 of each residue (can either be α or β) – may have major effect on the shape of the molecule which is a feature recognized by enzyme. 1.22. Some properties of oligosaccharides are describe in the table below: Sugars Sucrose Characteristics Energy provider in many organism Found in many fruits, roots, seeds and honey Lactose Animal energy source Found in milk Trehalose Energy source for insects Found in insect blood, yeast and Fungi Maltose Derived from starch and glycogen polymers Found in plant (starch), and animal (glycogen-energy storage- animal starch) Cellobiose Derived from cellulose polymers Cellulose is structural component of cell wall 1.23. Oligosaccharides are also found as part of glycoproteins and play a role in cell recognition/identity. Oligosaccharides form the blood group antigens. In some 45 PRT3402- Agricultural Biochemistry PJJ UPM / UPMET cells, these antigens are attached as O-linked glycans to membrane proteins. Alternatively, the oligosaccharide may be linked to a lipid molecule to form a glycolipid. These oligosaccharides determine the blood group types in humans. 46 PRT3402- Agricultural Biochemistry PJJ UPM / UPMET TOPIC 2: STRUCTURE AND FUNCTIONS POLYSACCHARIDES Main Points 2.1 Polysaccharides are polymers of monosaccharides and their derivatives and they are also known as glycans. Homopolysaccharides or homoglycan contains one kind of monosaccharide molecules while heteropolysaccharides or heteroglycancan have more than one kind of monosaccharide molecules. 2.2 The table below described some common polysaccharides, the monomeric units and the linkages that form the polymers. 2.3 Polysaccharides Monomeric units Linkages Glycogen Cellulose Chitin Amylopectin Amylose D Glucose D Glucose N-Acetyl-D-glucosamine D Glucose D Glucose 1>6 branches 1>4 1>4 1>6 branches 1>4 Linkages between the individual units require special enzymes to break them down. For example, the α1->4 linkages between glucose units in glycogen, amylose, and amylopectin, are readily broken down by all animals, but only ruminant animals (cows, horses, etc.) contain symbiotic bacteria with an enzyme (cellulase) that can break down the 1->4 linkages between individual glucose units in cellulose. 2.4 Polysaccharides differ from one another due to: - Component of monosaccharides - Length of their chains - Amount of chain branching also distinguished polysaccharides from protein, nucleic acids which occur only as linear polymers. 2.5 Functions of polysaccharides include: 1) Storage materials e.g. Starch, glycogen and dextran -metabolized to produce ATP 2) Structural components Chitin - skeletons of arthropods Cellulose – green plants 47 PRT3402- Agricultural Biochemistry 3) 2.6 PJJ UPM / UPMET Protective substances Starch is the polysaccharide storage materials in plants and exists in two different forms: α-amylose and amylopectin. Starch in nature consist of 10-30 % α-amylose and 70-90 % amylopectin. Cornstarch is made-up of 25% αamylose and 75% amylopectin. α-amylose is made up of D-glucose units with α(1→4) linkages. It is a linear polymer consisting of 300 to 3000 or more of glucose units. It is an energy storage material, insoluble in water and used as gelling agents. The structure of starch amylase is shown below: 2.7 Amylopectin is a polymer of highly branched chain of glucose units. Branches occur every 12-30 residues with an average branch length of 24-30 residues. The linear way of polymerization is through α(1→4) glycosidic bonds while the branching is through α(1→6) bonds as shown below. It is soluble in water and its uses include pastes, adhesives, and lubricants. 2.8 Glycogen is another storage polymer made from polysaccharides but it is mainly in animals unlike starch in plants. It is found mainly in the liver. The 48 PRT3402- Agricultural Biochemistry PJJ UPM / UPMET structure is similar to amylopectin but highly branched. The polymer has branches for every 8-12 glucose units. 2.9 Dextran is the storage polysaccharides found in yeasts and bacteria. It is made-up of straight chains of D-glucose units link by α(1→6) linkages, and it branches by α-1,3 linkages. In the medical field it is use to reduce blood viscosity. 2.10 Polysaccharide that functions as structural components include cellulose which is the main constituents of many plants and chitin which is the protective skeletons of arthropods. Cellulose is a linear polymer linked through glucose β(1→4) linkages. Cellulose is the most abundant natural polymer in nature, found in the cell wall of plant cells. It gives structural integrity and strength to the plants. The β (1→4) linkages of cellulose generate a planar structure and the parallel cellulose chains are linked together by a network of hydrogen bonds. This results in cellulose having a great mechanical strength but limited extensibility. Structure of cellulose 2.11 Chitin is a homopolymer of N-acetyl-β-D- glucosamine, a derivative of glucose with β,(1→4) linkages. It is the structural material in exoskeleton of many arthropods such as crabs, shrimps and molluscs. Chitin can be used for many purposes such as binder in dyes, fabrics, and adhesives. It can be used as fertilizer to improve crop yields and as surgical threads in surgical operations. 49 PRT3402- Agricultural Biochemistry 2.12 PJJ UPM / UPMET Saccharides can also be linked to non-sugar components. Proteoglycans are complexes of polysaccharides consisting of glycosaminoglycans and specific proteins. Peptidoglycans are heteroglycan chains linked to peptides. The repeating unit of peptidoglycans is a disaccharide composed of N-acetyl-Dglucosamine and N-acetylmuramic acid joined by a β-1,4 glycosidic bond. Short chains of amino acids (peptides) are linked as branch to the heteroglycans. Many of the peptidoglycans are present in bacterial cell wall. The structure and linkages is presented below. 50