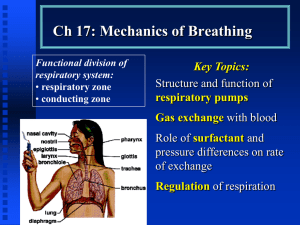
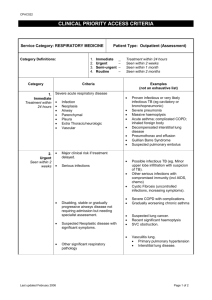
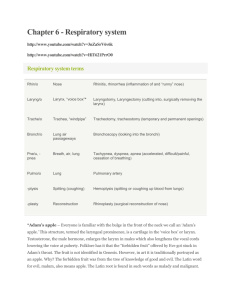
Respiratory notes BETA
advertisement

Giles Kisby GE Y1 Respiratory Respiratory: Spring Term: LECTURES: 1 Giles Kisby GE Y1 Respiratory Learning Outcomes – Year 1 (2013) Autumn Term: The main goal of this course is to help you develop a sound understanding of how the respiratory system achieves the vital function of gas exchange, and how this is perturbed in disease. The primary goal is to equip you with the knowledge base to apply basic respiratory science to clinical practice in the later years of your course, recognising that the practice of respiratory medicine is integrally related to the physiological processes you will cover over this term. We also hope to instill a finer appreciation of the scientific intricacies of the respiratory system. The course is structured in five overlapping themes: Theme one covers functional anatomy, and embryology. In the second theme, the essentials of gas exchange are understood through progression from mechanical considerations of gas transfer, delivery of blood to the alveolar capillary unit, and ventilation perfusion matching. Theme three explores the regulation of breathing both awake and asleep. In theme 4, we will focus on the consequences to normal physiology „when things go wrong‟ in disease states. Finally, in theme 5, physiology in extreme normal circumstances will be addressed as part of a review of the fundamentals covered within the course. At the end of the course, you will be able to the lung epithelium and the function of the cells contained within it. Describe the main muscles used in breathing and how these are utilised to generate different lung volumes. List the lung volumes that can be measured. alveolar ventilation the control of breathing and how this changes during sleep dyspnoea, chest pain. nd how they influence breathing. hypersensitivity, specifically asthma. physiological consequences of infection in healthy lungs 2 Giles Kisby GE Y1 Respiratory LECTURES Lecture 1: Respiratory System I (Dr Claire Shovlin, c.shovlin@imperial.ac.uk) This lecture should allow you ..... uscles that enable expiration and inspiration tilising concepts of surface tension, the Law of Laplace, and pulmonary surfactant. e difference between obstructive and restrictive airways disease Lecture 2: Respiratory System II (Dr Claire Shovlin, c.shovlin@imperial.ac.uk) At the end of this lecture you should be able provide three mechanisms that increase the rate of diffusion ace d the principle of haemoglobin and oxygen carriage Lecture3: Mechanisms of Breathing (Dr Claire Shovlin, c.shovlin@imperial.ac.uk) At the end of this lecture you should be able to • Recall the principal muscles associated with inspiration and expiration • Recall the anatomical basis of breathing, describing the two phases of respiration and the role of the thoracic cage and diaphragm and changes in the capacity of the thoracic cavity during these events. • Recall how contraction of inspiratory muscles causes the chest wall to expand and the lungs to enlarge • Recall how the chest wall contracts and lungs reduce in size • Explain what is meant by elastic recoil • Define compliance • Explain how pulmonary versus chest wall compliances can vary in various respiratory diseases • Explain the concept of surface tension and the Law of Laplace • Explain how pulmonary surfactant affects lung volume and airway patency 3 Giles Kisby GE Y1 Respiratory • Recall the relationship between alveolar and atmospheric gas pressures, airway resistance and airflow • Recall the factors that affect airway resistance centrally and peripherally • List the two major components that contribute to the work of breathing and explain how each may be altered in disease states • Recall the relationship between mechanical work and oxygen cost of breathing in normal individuals and patients with respiratory insufficiency • Recognise that respiratory muscles control air movement during other behaviours including, speech, laughter, coughing, sneezing and vomiting. Lecture 4: Respiratory Muscles (Dr. Kevin Murphy (kevin.murphy@imperial.ac.uk) At the end of this lecture you should be able to: additional non-respiratory actions of these muscles. to enlarge. lungs to reduce in size. Specifically: identify which will be active during quiet breathing, and which will be active when ventilatory demand is increased such as during exercise or during lung disease. will control air movement during other behaviours including, speech, laughter, coughing, sneezing and vomiting. Lecture 5: Lung Development (Dr Matthew Hind, m.hind@imperial.ac.uk) At the end of this lecture you should be able to understand and describe..... ital defects arise development al period Lecture 6: Pulmonary Circulation I. (Dr Claire Shovlin, c.shovlin@imperial.ac.uk) At the end of this lecture you should be able to: structure of the two ventricles of the heart. standing human. 4 Giles Kisby GE Y1 Respiratory recruitment and hypoxic vasoconstriction. disadvantage of this response in an adult suffering from chronic lung disease capillaries to lymphatics Lecture 7: Pulmonary Circulation II. (Dr Claire Shovlin, c.shovlin@imperial.ac.uk) At the end of this lecture you should be able to: may lead to this state. o the right side of the heart and the pulmonary circulation, o the viability of the lung tissue and o the implications for gas exchange. differences between normal, shunting, and dead space. Give one example of each. The last three concepts will be emphasized in a quiz. Lecture 8: Blood Gases (Dr Claire Shovlin, c.shovlin@imperial.ac.uk) At the end of this lecture you should be able to: following acid-base disturbances: o Acute respiratory acidosis o Acute respiratory alkalosis following renal compensation. rterial blood pH. PCO2 and Base Excess in the following acidbase disturbances: o Metabolic acidosis with respiratory compensation o Metabolic alkalosis with respiratory compensation -base status lead to alteration in ventilation and hence respiratory compensation. (i) Type I respiratory failure (ii) Type II respiratory failure, in each case after full renal compensation. Lectures 9 and 10: Regulation of Breathing (Professor Mary Morrell, m.morrell@imperial.ac.uk) metabolic homeostasis) and the behavioural controller (other needs such as speech) .Give five examples of respiratory or non-respiratory functions achieved by control of respiratory muscle activity 5 Giles Kisby GE Y1 Respiratory breathing, and structures in higher brain areas (suprapontine) that drive behavioural (nonautomatic) control of breathing. Describe how they can act independently or interact for control of the respiratory pump. tory control system (central and peripheral chemoreceptors, lungs, airways and chest wall) and describe the common motor outputs. , consider its role in breathing control and its clinical impact (also considered in lecture 11). and „congenital central hypoventilation syndrome‟ („ondines curse‟) in chemosensitivity and the apnoeic threshold led to central sleep apnoea. sleep apnoea. erbated by the sleep-related changes in the control of breathing; briefly explain why sleep is detrimental to these patients Lecture 11: Sensory Aspects of Respiratory Disease (Professor Fan Chung, f.chung@imperial.ac.uk) At the end of this lecture, in the indicated settings, you should be able to General research thophysiological basis of the respiratory symptoms cough, chest pain (and dyspnoea, covered elsewhere): Cough glottis. Briefly explain how this manouevre serves to i) protect the lungs from inhaled noxious materials and ii) clear excessive secretions from the lower respiratory tract stimulated to give rise to cough. Identify the neural pathways which transmit this afferent (sensory) information to the brain -ordinated neural activity that results in a cough. Identify the efferent (motor) neural pathways and the main muscle groups which produce cough. Chest pain receptors within the thoracic cavity that when stimulated give rise to chest pain. Identify the neural pathways that transmit this afferent neural information to the brain. 6 Giles Kisby GE Y1 Respiratory pain Dyspnoea and its measurement Lecture 12: Hypoxia (Dr Claire Shovlin, c.shovlin@imperial.ac.uk) At the end of this lecture you should be able to: and SaO2 (i.e. the O2 and CO2 dissociation curves), and factors affecting these curves with particular reference to oxygen uptake in the lung and the downloading of oxygen in the tissues. oxygen consumption; and the development of tissue hypoxia when delivery fails to meet demand with onset of anaerobic metabolism (lactic acid production) There is an accompanying computer aided learning quiz which you can run through with Dr Shovlin which will assist your understanding of: the „hyperpnoea‟ of exercise. -pulmonary capillary PO2 and PCO2. Explain the consequences of this for systemic arterial PO2 and PCO2. naemia) affects PaO2, PaCO2 and oxygen content. -enriched gas mixture in correcting any abnormalities associated with anaemia. g the effectiveness (or lack of it) of breathing an oxygen-enriched gas mixture in correcting any abnormalities associated with hypoventilation. NB: covered in Pulmonary Circulation II lecture Lecture 13: Diving (Dr Peter Wilmshurst) At the end of this lecture you should be able to -respiratory physiological principles are reinforced and modified by hyperbaric conditions Lecture 14: Lung function testing (Helium dilution and transfer factor) H Tighe (h.tighe@imperial.nhs.uk) 7 Giles Kisby GE Y1 Respiratory At the end of this lecture you should be able to e of measurement for lung volumes/capacities that can‟t be measure by simple spirometry trapping in COPD) gas diffusion across the alveolar membrane Lecture 15: Exercise Physiology. (Dr Luke Howard l.howard@imperial.ac.uk) At the end of this lecture you will appreciate and understand Lecture 16: Lung Cancer. (Dr Claire Shovlin c.shovlin@imperial.ac.uk) At the end of this lecture you should be able ..... susceptibility of the lung to particular carcinogens Lectures 17 and 18: Respiratory Failure I and II. Dr Umeer Waheed, Umeer.Waheed@imperial.nhs.uk, Dr Richard Stumpfl,Richard.Stumpfle@imperial.nhs.uk At the end of these lectures you should be able to -a gradient in Type 1 and 2 Respiratory Failure Lecture 19: Altitude and Air Travel. (Dr Robina Coker Robina.Coker@imperial.nhs.uk) This lecture will be delivered on line. At the end, you should be able to understand and describe.... poxic challenge tests Lectures 20 and 21: Airways Disease. (Dr Philip Ind p.ind@imperial.ac.uk) At the end of these lectures you should be able to understand and describe..... –distinction from restriction pirometry, peak flow, other measurements 8 Giles Kisby GE Y1 Respiratory -responsivenesss -COPD overlap d introduction to Guidelines for asthma and COPD PRACTICAL SESSION OBJECTIVES Practical 1: Lung volumes and spirometry Hannah Tighe (h.tighe@imperial.nhs.uk) values for lung volumes in a young healthy adult. predict these values. how these volumes may change during exercise. chronic restrictive lung disorder (2) severe chronic obstructive pulmonary disorder, and be able to give reasons for these changes. Practical 2: Airways resistance Hannah Tighe (h.tighe@imperial.nhs.uk) lung disease. females, increase with subject‟s height and decrease with age peaking at 20 years. Practical 3: Integrated exercise practical Dr. Luke Howard ( l.howard@imperial.ac.uk) Dr. Kevin Murphy (Kevin.murphy@imperial.ac.uk); techniques used to obtain the following cardio-pulmonary measurements taken during exercise : Ventilation, Heart rate, Blood Pressure, O2 consumption and CO2 production. SpO2. -respiratory response to an incremental work rate exercise test to exhaustion. disease , (iv) training. (eg field exercise) may be limited by breathlessness. 9 Giles Kisby GE Y1 Respiratory LECTURES: 07/11/13: Respiratory system I: Dr Shovlin Los (from booklet): Lecture 1: Respiratory System I (Dr Claire Shovlin, c.shovlin@imperial.ac.uk) This lecture should allow you ..... To gain an overview of the respiratory system To list the main functions of the respiratory system To describe the respiratory pump To review the bones and muscles that enable expiration and inspiration To understand the passive properties of the respiratory system To explain what is meant by elastic recoil and compliance. To explain the factors that keep the non cartilagenous airways and alveoli open, utilising concepts of surface tension, the Law of Laplace, and pulmonary surfactant. To describe the relationship between airway resistance and airflow. To describe the factors that affect airway resistance centrally and peripherally. To understand the difference between obstructive and restrictive airways disease Notes: - - - Aerobic respiration more efficient than anaerobic and therefore lungs needed for gas exchange (both O2 in and CO2 out) Lung functions: o O2 for tissue delivery o CO2 removal to regulate pH o Filtration (prob to catch emboli before reach brain) [nb filtration of particles in the air also occurs: big particles at nose/mouth; small particles at cilia of conducting zone (and resp bronchioles); small particles at alveolar macrophages of alveoli] Requirements for airflow: o Patent airways o Open alveoli o Elastic and compliant lung parenchyma o Sealed pleural space o Functional muscles & bones at chest walls Requirements for gas exchange: o High concentration gradient: Airflow (see above) 10 Giles Kisby GE Y1 Respiratory o o o - Pulmonary circulation; bloodflow maintained partial pressure difference is the driving force for O2 across the alveolar/pulmonary capillary barrier High surface area Greater in adult than newborn (even proportionally speaking) 23 divisions of the airways; total SA increases at each level to 130m2 at alveoli in adult Capillaries form dense network over the alveoli; give 115m2 total endothelial surface area Thin distance to traverse <0.5µm distance between air and blood (endothelium, epithelium and interstitial space) To achieve this the capillary width falls to barely above RBC width [Exchange is also improved by “matched blood flow and air delivery”] Eg if pneumonia affecting one part of the lung would want airflow to be diverted; and intrinsic (non-nervous, non-hormonal) system achieves this; see later (is the same as how before birth the blood is diverted to the placenta) Fick’s Law: o The diffusion coefficient of a gas (D) is a combination of the usual diffusion coefficient, which depends on molecular weight (see Chapter 1), and the solubility of the gas. The diffusion coefficient of the gas has enormous implications for its diffusion rate, as illustrated by differences in the diffusion rates of CO2 and O2. The diffusion coefficient for CO2 is approximately 20 times higher than the diffusion coefficient for O2; as a result, for a given arterial pressure difference, CO2 diffuses approximately 20 times faster than O2 o Several of the terms in the previous equation for diffusion can be combined into a single term called the lung diffusing capacity (DL). DL combines the diffusion coefficient of the gas and the thickness of the membrane (Dx). DL also takes into account the time required for the gas to combine with proteins in pulmonary capillary blood (e.g., binding of O2 to hemoglobin in red cells). In emphysema, for example, DL decreases because destruction of alveoli results in a decreased surface area for gas exchange. In fibrosis or pulmonary edema, DL decreases because the diffusion distance (membrane thickness or interstitial volume) increases. In anemia, DL decreases because the amount of haemoglobin in red blood cells is reduced (recall that DL includes the protein-binding component of O2 exchange). During exercise, DL increases because additional capillaries are perfused with blood, which increases the surface area for gas exchange. o Emphysema (from smoking): SA loss due to burst alveoli o Pneumonia, fibrosis: increased distance for diffusion (thickened walls – “alveolarcapillary block” o Pneumonia: 11 Giles Kisby GE Y1 Respiratory - - - caused by infection with viruses or bacteria The lungs quickly fill with fluid and become stiff. Stiffness gives restrictive problems with breathing Fluid gives “alveolar-capillary block” and can trigger vasoconstriction; a problem if the pneumonia is widespread The conducting zone: o Includes the nose, nasopharynx, larynx, trachea, bronchi, bronchioles, and terminal bronchioles. These structures function to bring air into and out of the respiratory zone for as exchange and to warm, humidify [important: prob to help maintain a moist surfactant layer], and filter the air before it reaches the critical gas exchange region. The respiratory zone: o Includes the structures that are lined with alveoli and, therefore, participate in gas exchange: the respiratory bronchioles, the alveolar ducts, and the alveolar sacs Compliance: o Describes change in volume for a given change in pressure 12 Giles Kisby - GE Y1 Respiratory o V. Low for pulmonary fibrosis o V. High for emphysema Airway defences by epithelial cells ‘pseudostratified columnar epithelium’: o 80% of conducting airway cells: ciliated bronchial epithelial cells: At conducting airway cells and respiratory bronchioles: ciliated cells dominantly occupy that of the trachea-bronchus (while Clara cells are dominantly distributed at the epithelium of the bronchioles; see diagram) Beat to reduce entry to lower airways; function lost in smoking / infections o 20% of conducting airways: goblet cells; They are found inside the trachea, bronchus, and larger bronchioles in respiratory tract Secrete mucus: Adhesive for incoming particles Antimicrobial Antioxidant o Also: clara cells: Metabolise chemicals coming through; involved in metabolism of inhaled foreign compounds Clara cells are dominantly distributed at the epithelium of the bronchioles (while ciliated cells dominantly occupy that of the trachea-bronchus; see diagram) o Alveolar Type II pneumocytes: At alveoli Produce surfactant which has immune properties and used to prevent alveolar collapse (see later) o Phagocytic cells: Immune system function Eg. Alveolar macrophages [at alveoli], polymorphonuclear neutrophils - Types of cell in the lung: o o o Trachea: Basal Cells Ciliated Cells Glands Goblet Cells Neuroendocrine Airway: Ciliated Cells (80% number) Clara Cells Neuroendocrine Goblet Cells (20% number) Alveolus: Type 1 Cells: 90% of SA, equal number as type 2, thin for gas exchange 13 Giles Kisby GE Y1 Respiratory [ie Alveolar Type I pneumocytes] Simple squamous epithelium Type 2 Cells: 10% of SA, equal number as type 1, produce surfactant [ie Alveolar Type II pneumocytes] Cuboidal epithelium - Lipofibroblasts Myofibroblasts [also prob phagocytes] Airway structure: o See diagram below: o Nb only in areas of the lung with smooth muscle present can sympathetics and parasymapathetics give dilation/constriction [ie not at alveolar sacs; most plentiful in conducting zone] 14 Giles Kisby GE Y1 Respiratory 07/11/13: Respiratory system II: gasses, Pressure and Volumes: Dr Shovlin Los (from booklet): Lecture 2: Respiratory System II (Dr Claire Shovlin, c.shovlin@imperial.ac.uk) At the end of this lecture you should be able ’s Gas Law and apply at sea level and Everest nderstand the principle that PaCO2 is the primary driver of ventilation Notes: - Gas Laws: o Dalton’s Law: Total pressure = sum of the partial pressures o Air total composition (at any altitude): 21% oxygen, 78% nitrogen, 1% other o p(O2) will always be 21% of the total pressure of dry air at that altitude (but its pressure contribution in kPa/mmHg will vary with altitude) o Total pressure at sea level = 101kPa so p(O2) = 21.2 kPa (159mmHg) o Note that pressures may be given in kPa, mmHg, cmH2O, atm o To factor in the influence of humidity: 15 Giles Kisby GE Y1 Respiratory FRC = Functional residual capacity; RV = residual volume - Tidal and reserves: o Tidal volume usually 0.5L but all other values show large variation between people o Note that not all intrathoracic air is available for gas exchange; exchange can only occur in functional alveoli: Anatomical /anatomic deadspace volume is volume of intra-thoracic air in a tidal volume minus intr-alveolar air The anatomic dead space is the volume of the conducting airways, including the nose (and/or mouth), trachea, bronchi, and bronchioles. It does not include the respiratory bronchioles and alveoli. The volume of the conducting airways is approximately 150 mL: is the first air expired [important to consider if sampling breath etc] At the end of expiration the conducting airways are filled with alveolar air; that is, they are filled with air that has already been in the alveoli and exchanged gases with pulmonary capillary blood. With the inspiration of the next tidal volume, this alveolar air is first to enter the alveoli, although it will not undergo further gas exchange. The next air to enter the alveoli is fresh air from the inspired tidal volume (350 mL) which will undergo gas exchange Physiological /physiologic deadspace volume is is volume of intra-thoracic air in a tidal volume minus intr-alveolar air of functional alveoli only Ie includes the anatomic dead space of the conducting airways plus a functional dead space in the alveoli [ventilated alveoli that do not participate in gas exchange]. In normal persons, the physiologic dead space is nearly equal to the anatomic dead space [due to good regional V/Q matching] Volume of the physiologic dead space is estimated with the following method: If physiologic dead space is zero, then PECO2 will be equal to alveolar PCO2 (PACO2). However, if a physiologic dead 16 Giles Kisby GE Y1 Respiratory space is present, then PECO2 will be “diluted” by dead space air and PECO2 will be less than PACO2 by a dilution factor. [nb PaCO2 = PACO2] o The Minute ventilation (= minute volume = total ventilation = pulmonary ventilation) is the mlmin-1 at rest. Ie volume of gas inhaled (inhaled minute volume) or exhaled (exhaled minute volume) from a person's lungs per minute Around 12 breaths per min at rest so is 12*500 = 6000mlmin-1 o The alveolar ventilation is the volume of air moving into or out of the functioning alveoli per min at rest (usually in mlmin-1): Ie Is 12 * (500 – anatomical deadspace volume – alveolar deadspace volume) = 12 * (500 – physiological deadspace) o Key values: [from sea level atm 100KPa = 760mmHg downward] inhaled dry air p(O2) = 21.2 kPa = 160mmHg inhaled dry air p(CO2) = negligible Humidified tracheal air on inspiration p(O2) = 20 kPa = 150mmHg Humidified tracheal air on inspiration p(CO2) = negligible It is assumed that the air becomes fully saturated with water vapour. At 37°C, PH2O is 47 mm Hg (=6.3kPa) Values can be calculated from the dry values using the relevant equation Alveolar p(O2) = 13.3 kPa =100 mmHg arterial p(O2) = 13.3 / 13kPa = 98/100 mmHg [slight decrease due to physiologic shunts] Reason for low p(O2): due to dilution with high CO2 levels (ie prior to gas exchange) and due to the gas exchange events themselves (ie loss of O2: equilibrates with blood in healthy person; and further CO2 dilution) Alveolar p(CO2) = 5.3kPa = 40mmHg arterial p(CO2) = 5.3kPa = 40mmHg [slight increase due to physiologic shunts] venous p(O2) = 5.3kPa = 40mmHg venous p(CO2) = 6.1kPa = 46mmHg 17 Giles Kisby GE Y1 Respiratory o o o Shunts: Physiologic shunt. About 2% of the cardiac output normally bypasses the alveoli—there is a physiologic right-to-left shunt. Part of the physiologic shunt is the bronchial blood flow, which serves the metabolic functions of the bronchi. The other component of the shunt is the small amount of coronary blood flow that drains directly into the left ventricle through the thebesian veins and never perfuses the lungs. Small physiologic shunts are always present, and PaO2 will always be slightly less than PAO2 (V. small Aa difference). Shunt V/Q defects add to the magnitude of physiologic shunt and increases Aa difference. Right-to-left shunts. Shunting of blood from the right heart to the left heart can occur if there is a defect in the wall between the right and left ventricles. In a right-to-left shunt, hypoxemia always occurs. Cannot be corrected by having the person breathe a high O2 gas (e.g., 100% O2) “Aa gradient” [ie A=alveolar, a = arterial] Increases with: V/Q defect [inc Diffusion defects (e.g., fibrosis, pneumonia) and Right-to-left shunt] Age o Due to lower arterial blood O2 levels caused by poorer gas exchange primarily due to less elastic lungs Normal in: High altitude (dec PIO2) Hypoventilation (dec PAO2) pa(CO2) [ie p(CO2) of the arteries] leaving the lungs is the main driver of ventilation via pH changes: looked at in detail in later lecs chemoreceptors at carotid body and aortic arch respond to pH, pa(CO2) and pa(O2) of blood (relevance mainly to pa(O2) but relatively insensitive to pa(O2) as can drop greatly without ill effect due to being at the flat part of 18 Giles Kisby GE Y1 Respiratory o oxygen dissociation curve; before this occurs usually CO2 signals: quickly accumulates and signals to raise ventilation so this acts as the main driver for increased ventilation) If arterial PO2 is less than 60 mm Hg, the breathing rate increases in a very steep and linear fashion. In this range of PO2, chemoreceptors are exquisitely sensitive to O2 o Such hyperventilation can occur at altitude; at first gives inc in pH due to breathing off extra CO2 than what is necessary (to try to gain sufficient O2 which may only be at 60mmHg) – this inc pH gives ventilation dec via dec pH ventilation signalling but then within a few days the alkylosis is compensated for via HCO3- excretion from body so hyperventilation resumes o The initial phase of ascent to high altitude is associated with a constellation of complaints, including headache, fatigue, dizziness, nausea, palpitations, and insomnia. The symptoms are attributable to the initial hypoxia and respiratory alkalosis, which abate when the adaptive responses are established. Detection of changes in PCO2 by the peripheral chemoreceptors is less important than detection of changes in PCO2 by the central chemoreceptors. chemoreceptors of medulla respond to pH and p(CO2) of CSF blood-brain barrier is relatively impermeable to H+ and HCO3 CO2 passes through BBB and brain-CSF barrier In the CSF, CO2 is converted to H+ and HCO3- and is detected by the chemoreceptors pa(CO2) = ~5.3kPa is normal [important to know] (45mmHg) Acceptable: 4.7-6 kPa 2kPa dizziness (due to high RR) 12kPa drowsiness, life threatening If the patient's arterial partial pressure of carbon dioxide (PaCO2) is greater than 6 kPa: - refer them urgently to a specialist respiratory service (to be seen within 1 week) Other receptors: Lung stretch receptors Joint and muscle ergoreceptors [pre-emptive response] Irritant receptors J receptors pa(O2) governs O2 delivery to tissues: Henry’s law: rate gas dissolves in an inert liquid is proportional to its partial pressure 19 Giles Kisby GE Y1 Respiratory At normal pa(O2) only 0.3mls O2 /100mls [ie PaO2 x solubility in ml/100ml/pressure unit] of blood dissolves [2% of the total O2 content of blood] – this is not enough; haemoglobin is used to aid O2 capacity of blood – carries 20mls / 100mls [ie 1.34ml/g x 15g/100ml – see pukka notes] of blood [98% of the total O2 content of blood] Ie total O2 in blood = O2 dissolved + O2 on haemoglobin Haemoglobin: amount of O2 bound vs p(O2) is a sigmoidal curve due to cooperativity of O2 binding Haemoglobin leaving lungs is usually ~100% saturated At rest, normal conditions, in the tissues saturatin is approximately 40 mm Hg: hemoglobin is only 75% saturated and the affinity for O2 is decreased Haemoglobin: not 100% O2 delivered to tissues but large amount is due to shift of sigmoidal curve due to CO2 presence, etc P50; 23DPG; temp; CO2; pH; Bohr; Haldane; CO [left shift (Those heme groups not bound to CO have an increased affinity for O2) and change shape (decreases the number of O2-binding sites available on hemoglobin); carboxyhaemoglobin; carbaminohaemoglobin Methemoglobin [Fe3+ doesn’t bind O2; nitrates, sulphonamides; methaemoglobin reductase]; Fetal hemoglobin (hemoglobin F); Hemoglobin S (sickle cell disease) Delivery to tissues: o Anemia, trauma, low HR gives reduced O2 delivery to tissues Disease: Types of breathing problem: i. Airflow obstruction: is only concerned with airway issues; eg asthma ii. Airflow restriction: concerned with: Patent airways Open alveoli Elastic and compliant lung parenchyma Sealed pleural space Functional muscles & bones at chest walls iii. Bloodflow obstruction: emboli iv. Nervous: Polio can cause phrenic nerve damage and therefore breathing difficulties 20 Giles Kisby GE Y1 Respiratory 08/11/13: Mechanisms of breathing: Dr Shovlin Los (from booklet): Lecture3: Mechanisms of Breathing (Dr Claire Shovlin, c.shovlin@imperial.ac.uk) At the end of this lecture you should be able to Recall the principal muscles associated with inspiration and expiration Recall the anatomical basis of breathing, describing the two phases of respiration and the role of the thoracic cage and diaphragm and changes in the capacity of the thoracic cavity during these events. Recall how contraction of inspiratory muscles causes the chest wall to expand and the lungs to enlarge Recall how the chest wall contracts and lungs reduce in size Explain what is meant by elastic recoil Define compliance Explain how pulmonary versus chest wall compliances can vary in various respiratory diseases Explain the concept of surface tension and the Law of Laplace Explain how pulmonary surfactant affects lung volume and airway patency Recall the relationship between alveolar and atmospheric gas pressures, airway resistance and airflow Recall the factors that affect airway resistance centrally and peripherally List the two major components that contribute to the work of breathing and explain how each may be altered in disease states Recall the relationship between mechanical work and oxygen cost of breathing in normal individuals and patients with respiratory insufficiency Recognise that respiratory muscles control air movement during other behaviours including, speech, laughter, coughing, sneezing and vomiting. Notes: - - Posteriorly the oblique fissure starts at T4 Inspiration is louder than expiration Pneumonia gives “bronchial breathing” with expiration as loud as inspiration At the Functional residual capacity (FRC) the outward force of the chest wall trying to expand to its more favourable, larger state is exactly balanced by the tension of the lungs trying to contract to their more favourable, smaller state. o Inspiratory and expiratory muscles give deviations from this volume o If thorax opened, chest would expand by 600-1000mL Zones of apposition: lateral zones of the diaphragm that are directly against the inner surface of the rib cage 21 Giles Kisby - - - - GE Y1 Respiratory Note that decent of diaphragm, as well as expanding the intrathoracic volume directly, also exerts an outward force on the lower abdominal wall (presumably due to resistance of abdominal organs) to cause rib elevation and expansion and further expand the intrathoracic volume Oesophageal pressure changes follow pleural pressure changes Boyle’s law states that P x V is constant at a given temperature o P1V1 = P2V2 Transmural pressure is calculated as alveolar pressure minus intrapleural pressure. If transmural pressure is positive, it is an expanding pressure on the lung, and the arrow points outward. If transmural pressure is negative, it is a collapsing pressure on the lung, and the arrow points inward (does not ever occur in healthy state) During inspiration, intrapleural pressure becomes more negative than at rest: o As lung volume increases, the elastic recoil of the lungs also increases and pulls more forcefully against the intrapleural space, and o airway and alveolar pressures become negative. Tidal breathing gives smooth sine waves: 22 Giles Kisby - - GE Y1 Respiratory Compliance = Δvolume/ΔPressure o High in healthy lungs (but not extremely high as for emphysema) o The external force required to deform the structure (ie refers to inspiration) o Compliance Increases with age Elasticity = ΔPressure/Δvolume = 1/compliance o High in healthy lungs o Property of matter that causes it to return to its resting shape after deformation (ie refers to expiration) o Determines the volume left in the lungs after a normal tidal exhalation (ie the FRC) o Elasticity decreases with age so FRC rises and potentially more input from expiratory muscles instead of relying on passive exhale - The thin rubber band has the smaller amount of elastic “tissue”—it is easily stretched, and is very distensible and compliant. The thick rubber band has the larger amount of elastic “tissue”—it is difficult to stretch and is less distensible and compliant. Furthermore, when stretched, the thick rubber band, with its greater elastance, “snaps back” with more vigor than the thin rubber band does. - Floppy balloon/emphysema = high compliance, low elastic resistance/recoil (expiratory muscles have to work harder – passive insufficient to expel air [these will also have to work harder for asthma too (but this is for reasons of airflow obstruction not airflow restriction)] [will also have to work harder with age]) Stiff balloon/pulmonary fibrosis = low compliance, high elastic resistance/recoil (inspiratory muscles have to work harder) [ie regardless of which type of disease will give larger overall energy cost of expiration] - - Below diagram shows volume changes with changing transmural (= transpulmonary) pressure (difference in pressure across the alveolar wall); the dotted lines just indicate the favourability of the position at that pressure of the chest wall/lung o Ptp = Palv - Pip. Where Ptp is transpulmonary pressure, Palv is alveolar pressure, and Pip is intrapleural pressure o If 'transpulmonary pressure' = 0 (alveolar pressure = intrapleural pressure), such as when the lungs are removed from the chest cavity or air enters the intrapleural space (a pneumothorax) o Under normal physiological conditions (ie tidal breathing) the transpulmonary pressure is always positive; intrapleural pressure is always negative and relatively large, while alveolar pressure moves from slightly positive to slightly negative as a person breathes. o Eg. At FRC the chest wall lines are equidistant from the combined line indicating balance of the forces; collapsing force equal to the expanding force [ie muscle actions are not present on the plot but this is the only position at which they are not active – elsewhere are required to overcome the collapsing/ expanding forces] 23 Giles Kisby GE Y1 Respiratory o o o eg above FRC the muscles are overcoming the net collapsing force due to lung collapse > chest wall expansion force or at higher pressures them both exerting collapsing florce. Similarly below FRC there is a net expansion effect due to chest expansion > lung collapse but the muscles are overcoming this to hold the lungs in this position in fact on the 2nd graph an applied pos/neg pressure is used to hold the positions NB her diagram prob bad as shouldn’t be getting negative transmural pressures (would indicate a collapsing effect) The slopes of the relationships for inspiration and expiration are different, a phenomenon called hysteresis. Usually, compliance is measured on the expiration limb. On the inspiration limb, one begins at low lung volume where the liquid molecules are closest together and intermolecular forces are highest; to inflate the lung, one must first break up these intermolecular forces. On the expiration limb, one begins at high lung volume, where intermolecular forces between liquid molecules are low; one needn’t break up intermolecular forces to deflate the lung. Gradient is proportional to compliance: The compliance of the chest wall alone is approximately equal to the compliance of the lungs. However, the compliance of the combined lung and chest-wall system is less than that of either structure alone (like a balloon in a balloon) [nb the combined compliance curve changes with diseases] 24 Giles Kisby GE Y1 Respiratory 25 Giles Kisby - - - GE Y1 Respiratory Emphysema (increased lung compliance). o Emphysema is associated with loss of elastic fibers in the lungs. o Reduced collapsing force at a given volume o Thus, the combined lung and chest-wall system seeks a new higher FRC, where the two opposing forces can be balanced o breathe at higher lung volumes (in recognition of the higher FRC) and will have a barrel-shaped chest. Fibrosis (decreased lung compliance) o stiffening of lung tissues o Increased collapsing force at a given volume o Thus, the combined lung and chest-wall system seeks a new lower FRC, where the two opposing forces can be balanced o breathe at lower lung volumes Keeping the alveoli open during inspiration: o the alveolar pressure is negative during this period so would think they would want to collapse o Alveoli can be considered as gas bubbles in a liquid so can use the Law of Laplace Law of Laplace states that P = 2T/R for such a bubble to be stable and to stop collapsing, where P = pressure at which the bubble is stable (want it to be low), T=tension (ie low allows for lower pressure) and R=radius (ie high allows for lower pressure) Hence smaller alveoli require larger pressures to remain open and should be the first to collapse This problem is avoided by using a higher surfactant concentration at smaller alveoli; the surfactant (produced by type II pneumocytes) lowers surface tension to allow alveoli stability at reduced pressures and by using higher surfactant concentrations at smaller alveoli further reductions in surface tension are offered to compensate for their greater propensity to collapse. For a given drop in pressure, as alveoli start to shrink (reducing R, radius) the tension (T, surface tension) falls faster, therefore will quickly reach a new set 26 Giles Kisby GE Y1 Respiratory o - of R&T values that via the equation match the required P value which was previously too high. Surfactant info: Is dipalmatoylphosphatidyl choline mainly Has the lowest surface tension of any biological substance Intermolecular forces between the DPPC molecules break up the attracting forces between liquid molecules lining the alveoli Prevents collapse Increases compliance Form relatively late in gestation; premature babies without it have breathing difficulties: “(neonatal) respiratory distress syndrome”; glucocorticoids can be given to mother to encourage type II pneumocyte cells of child to produce surfactant Airflow resistance [must be overcome in active inspiration, passive expiration, active expiration]: o Components: Elastic resistance of lungs (ie dependant on how elastic/compliant the lungs are) Flow Resistance in upper airways and tracheobronchial tree to airflow (turbulent / laminar flow is relevant) (Poiseuille’s equation is relevant) (airway patency is relevant) Frictional resistance of tissues in lung parenchyma and chest wall sliding over each other o Effect of flow type: ΔP α airflow for laminar flow ΔP α airflow2 for turbulent flow [ie greater pressure required for given flow] Reynolds number = (2 * Radius * Density of gas * velocity of gas) / gas viscosity The number is used to predict which type of flow will occur High numbers indicate turbulent flow, low numbers indicate laminar flow Ie wide airways, branch points (inc radius) and high velocities are associated with turbulent flow Helium can be used to extend life a small amount by reducing density but not viscosity of the air o Poiseuille’s equation: Airflow = ΔP / R R = (8Ln)/(πr4) Therefore: Airflow = (ΔP πr4)/8Ln Consequence is that while smaller airways individually have higher airflow resistance, many in parallel give a low overall resistance Quiet breathing: 27 Giles Kisby GE Y1 Respiratory o o Airflow resistance is greatest in the upper airways and low in the small peripheral airways [specifically the (mediumsized)bronchi are the sites of highest airway resistance] However, towards the end of forced expiration: o As lung volume decreases, airways narrow, esp peripherally and the main contribution to airflow resistance moves peripherally Airway patency: Central airways: Held open by cartilage Factors affecting resistance: o Symp/para action on smooth muscle modulates width (bronchioconstriction/dilation) o Breathing: will give some changes to width via width changes o Foreign bodies o Mucus hypersecretion Peripheral airways: Are connected to the alveolar network and therefore held open by the same forces holding open the alveoli (as described by Law of Laplace Factors affecting resistance: o Symp/para action on smooth muscle modulates width (bronchioconstriction/dilation) o Breathing: will give some changes to width via width changes o Emphysema: reduced “radial traction” – parenchyma fails to hold the airways open: choke points are more likely to occur (see later) [ie despite the usually beneficial effect of high lung volumes on radial traction] o Inflammatory fluid / edema [inc stiffness and displaces air] Some key determinants of Airway Resistance: Autonomic nervous system: sympa and para Lung volume. Changes in lung volume alter airway resistance because the surrounding lung tissue exerts radial traction on the airways. High lung volumes are associated with greater traction, which decreases airway resistance. Viscosity of inspired air Leukotrienes: from arachidonic acid metabolism; airway constriction [are metabolised at lung to lower level] Disease states NB O2, Thromboxane A2, prostacyclin (prostaglandin I2) are vasoactive so not relavant here 28 Giles Kisby GE Y1 Respiratory o Investigation by following airflow rates: [see graphs below] Moving clockwise from extreme right side of the graph patient is initially at the end of a forced exhale. Note that the Y-axis is flow rate; all inspiration is effort dependant (ie can be affected by patient thoughts); flow rate falls to 0 at end of inhale Patient asked to breath out as fast as possible; early part is effort dependant and is utilising the forced expiration muscles However because the exhalation was so fast the passive exhalation has yet to occur (ordinarily would happen first!); this is passive and so effort independent (can’t be falsified by patient) In healthy person this last section is a continuation of the parabola into a straight line (as below): ie airway compression continues to be the limiting factor giving smooth change in flow rate If airway obstruction there will be a “sagging” of the line first (cannot passively maintain that airflow ejection rate due to block – block as well as airway compression is influencing the flow rate change) then a lower gradient loss of airflow 29 Giles Kisby GE Y1 Respiratory o o Bronchial movements and choke points: Note: at start of inspiration negative intrapleural pressure gives some bronchial expansion Note: during forced expiration, positive intrapleural pressure gives partial bronchial collapse between cartilages Choke points can develop in disease states; during forced exhalation the positive pressure magnitude reduces from the alveoli to the mouth but the pleural pressure is constant throughout Result is regions where the pleural pressure exceeds the pressure in the airways; the choke point forms at the point furthest to the alveoli where this condition is true; ie transmural pressure is negative here Air trapping behind the blockade occurs; blockade typically at 2nd / 3rd generation airways By lip pursing during their forced exhalation the patient is able to move the EPP (equal pressure point) (choke point) into the mouth as it gives raised airway pressures ‘upstream’ Pressure-flow relationships: [ie here are looking at flow vs pressure graphs which are different to the flow vs volume graphs so results give diff info] At high lung volume & expiration: Flow increases with increasing effort FEV1/VC should be more than 80% (ie for fastest possible exhale: forced expiratory volume in one second divided by vital capacity) o if not then obstruction likely to be present o ie would indicate a problem with the airways At low lung volume & expiration: Flow does not increase with increasing effort once a maximum is reached; thought to be because of airway compression in a healthy individual (note that this cannot be compared to the airflow vs volume graphs because is not for a cycle – is just the results at set applied transpulmonary pressures) [this graph is for the flow changes of a normal individual at three levels of lung inflation; high, mid, low lung inflation] 30 Giles Kisby - GE Y1 Respiratory in a normal person, FEV1/FVC is approximately 0.8, meaning that 80% of the vital capacity can be expired in the first second of forced expiration (see Fig. 5-6A). o In a patient with an obstructive lung disease such as asthma, both FVC and FEV1 are decreased, but FEV1 is decreased more than FVC is. Thus, FEV1/FVC is also decreased, which is typical of airway obstruction with increased resistance to expiratory airflow (see Fig. 5-6B). o In a patient with a restrictive lung disease such as fibrosis, both FVC and FEV1 are decreased, but FEV1 is decreased less than FVC is (or indeed may increase). Thus, in fibrosis, FEV1/ FVC is same or actually increased 31 Giles Kisby GE Y1 Respiratory 08/11/13: Respiratory Muscles: Dr. Kevin Murphy Los (from booklet): Lecture 4: Respiratory Muscles (Dr. Kevin Murphy (kevin.murphy@imperial.ac.uk) At the end of this lecture you should be able to: Identify the principal muscles associated with inspiration and expiration. Understand the additional non-respiratory actions of these muscles. Understand how contraction of inspiratory muscles causes the chest wall to expand and the lungs to enlarge. Understand how contraction of expiratory muscles causes the chest wall to contract and the lungs to reduce in size. Recognise that these muscles will be differentially activated during different breathing states. Specifically: identify which will be active during quiet breathing, and which will be active when ventilatory demand is increased such as during exercise or during lung disease. In addition to their primary role in maintaining alveolar ventilation, know that respiratory muscles will control air movement during other behaviours including, speech, laughter, coughing, sneezing and vomiting. Notes: - Inspiratory muscles: 32 Giles Kisby GE Y1 Respiratory Inspiration Quiet Augmented Expiration Quiet Augmented Resisted (eg in speech after large inhalation) Diaphragm mainly (others to a small degree) Diaphragm No muscles (passive: elastic recoil gives return to FRC) External oblique Sternocleidoma stoid Internal oblique Scalene Transversalis External intercostals Rectus abdominis Parasternal muscles Internal intercostals Diaphragm (to retard flow) 33 Giles Kisby GE Y1 Respiratory 34 Giles Kisby GE Y1 Respiratory 08/11/13: Lung Development: Dr Matthew Hind Los (from slides): - Understand that lung development is a continuous process [continues well after birth] Describe the stages of lung development Think about the plasticity of lungs Disorders during lung development can lead to lung diseases Can learn about lung development by looking at flies Many of the underlying mechanisms remain unknown Dramatic changes occur at birth Notes: - Gas exchange passive, dependant on Sa (achieved by high lung network complexity), thinness, conc grad - Types of cell in the lung: o o o Trachea: Basal Cells Ciliated Cells Glands Goblet Cells Neuroendocrine Airway: Ciliated Cells (80% number) Clara Cells Neuroendocrine Goblet Cells (20% number) Alveolus: Type 1 Cells: 90% of SA, equal number as type 2, thin for gas exchange [ie Alveolar Type I pneumocytes] - Type 2 Cells: 10% of SA, equal number as type 2, produce surfactant [ie Alveolar Type II pneumocytes] Lipofibroblasts Myofibroblasts [also prob phagocytes] 5 morphological stages of lung development: [need to know about each stage – see below] [6162636 weeks] 35 Giles Kisby GE Y1 Respiratory NB The foregut is the anterior part of the alimentary canal, from the mouth to the duodenum at the entrance of the bile duct. • • Embryonic - (day 22- 6 weeks) including lung bud formation • Occurs at day 22 human, mouse E9 • early in dev gastrulation occurs to give a tube (effectively mouth to anus) • Foregut endoderm invades splanchnic mesoderm (ie the lung is derived from the foregut (as is pancreas, etc)) • Lateral plate mesoderm is a type of mesoderm that is found at the periphery of the embryo. It will split into two layers, the somatic layer/mesoderm and the splanchnic layer/mesoderm: • The somatopleuric layer forms the future body wall. • The splanchnopleuric layer (splanchnic mesoderm) forms the circulatory system and future gut wall. • Larngotracheal groove moves in caudocranial direction (ie upwards) [The laryngotracheal groove is a precursor for the larynx and trachea] • Day 26-29 lung tube bifurcates into left and right bronchial buds • By 4.5 weeks the lung forms 5 saccules, the secondary bronchial buds: 3 right 2 left (ie become the lobes of the lung in the adult) • By continuous dichotomous branching upto the end of the 6th week the tertiary bronchial buds form – the bronchopulomonary segments of the mature lung • Branching morphogenesis and patterning occurs • Epithelial/mesenchymal signalling – factors released from mesenchyme act as cues for the developing lung bud • endoderm patterned by the mesenchyme – [Proof: remove the mesenchyme, tube dies; foreign mesenchyme eg somatic or mesonehric keeps tube alive but no branching; tip mesenchyme can induce branching in proximal endoderm] • FGF10 induces branching (ie factors from the mesenchyme are important in triggering lung branching) but not exclusively; chicken FGF is the same as mouse FGF but induces a different pattern of branching so other factors must be important Pseudoglandular (6 –16 weeks) • 7 weeks lung looks like a primitive gland; airways lined by columnar epithelium separated by thick undifferentiated mesenchyme • 65-75% of bronchial branching occurs between 10 and 14 weeks • Branching of airways is complete at the end of this phase • Epithelial cells are loaded with glycogen • Cilliated cells and goblet cells appear • Surfactant proteins first appear • Mesenchyme differentiates into cartilage and smooth muscle 36 Giles Kisby GE Y1 Respiratory • • • Cannalicular (16-26 weeks) [only after 22-24 weeks when this is nearly complete is breathing possible = min survival requirement] • Characterised by appearance of acini [cluster of cells that resembles a many-lobed "berry"] consisting of airway stem and a spray of tubules arranged as a cluster surrounded by loose mesenchyme – ie will become the alveoli eventually • Mesenchyme becomes riddled with capillaries becoming canalised • Differentiation of type II and type I cells. • Type II cells accumulate lamellar bodies (surfactant) • Proximal bronchiolar (Clara) cells accumulate CCSP • 26-28 weeks, human lung can support some gas-exchange especially if supported by surfactant (exogenous surfactant can be sprayed in) • Antenatal steroids given to mum induce surfactant synthesis and induce mesenchymal thinning and prevent RDS (BPD) Saccular (26/36 weeks) • Distal airspaces form terminal clusters of widened airspaces called saccules (will become the alveoli eventually) and there is expansion of airspace volume • Terminal sacs give rise to three generations of alveolar duct [Alveolar ducts are tiny ducts that connect the respiratory bronchioles to alveolar sacs] and one generation of alveolar sacs • As a result of the expansion of airspace volume the interstitum is compressed and its volume falls. • The capillaries become close together and the walls of the airspaces contain a double capillary network Alveolar (36 weeks -??? [ie evidence that further alveolar development can occur into puberty]) • Alveolar stage species dependant (ie varies between species when it occurs) • Elastin is involved in contracting to pinch sections into alveoli • Depends on oxygen requirements at birth • Guinea pigs, sheep and deer septate in utero • Rats mice and human largely postnatal • Humans 26 weeks – 18 months • Mice P4 – P14 37 Giles Kisby GE Y1 Respiratory [Pharmacology: The effects of ACh are relatively weak on nicotinic receptors so anticholinesterases have more potent effects at muscarinic receptors because raising ACh conc at nicotinics will proportionally give far less of a response than at muscarinic receptors] - - - Comparative biology: o genes that control fly respiratory development also control lung development in mouse and human o Mice have a highly manipulated genome and lots of molecular biology reagents that we can use to ask specific questions about developmental processes o Other models include rat, sheep, pig and baboon - different advantages Is lung disease lung development gone wrong? o Increasing evidence that early life events alter susceptibility to disease o Low birthweight associated with low FEV1 o Maternal smoking and asthma o Gene environment interactions and epigenetics o Genome, transcriptome, proteome and metabolomics o Stem cells as a causes of disease eg lung cancer or fibrosis o Impaired alveologenesis resulting in fewer alveoli, a reduced Sa and susceptibility to age related lung decline/emphysema Can we exploit developmental cues as novel therapy? o Retinoic acid has been demonstrated to restore SA in animal models of emphysema possibly by inducing regeneration (systemic retinoic acid has shown alveoli-regenerative action in models; steroids/elastase used to cause damage with RA giving regeneration; could equate to regeneration of emphysema damage in humans where alveoli lost) o HGF demonstrated to induce lung regeneration 38 Giles Kisby GE Y1 Respiratory o o o o Stem cells potentially could restore a lacking progenitor population with the correct microenvironment differentiate into functional lung tissue Understanding how airway remodelling occurs might reveal blocking strategies for airway conditions such as asthma Lung fibrosis seems to be driven by abnormal developmental signals can we switch them off? Ex-vivo lung perfusion systems with human donor lungs and artificial blood and air pumps can be used in such research to aid investigation. QUIZ important facts: [well worth doing again during revision – also, any quizlet exercises?] - Diffusion of gases across the alveolo-capillary membrane is very efficient. As a result the PO2 and PCO2 of the blood leaving a pulmonary capillary will be almost identical to that in the adjacent alveoli. - Although it is a bit of an oversimplification, you won't go far wrong if you assume that the appropriate level of ventilation is that which results in an arterial PCO2 of 5.3 kPa. o partial pressure of oxygen vs amount of oxygen in the blood: - The partial pressure of oxygen in systemic arterial blood (ie here are considering the amount dissolved – so Hb not relevant) in a healthy individual is determined by alveolar PO2 (in accordance with Henry’s Law) - but the amount of oxygen in the blood (the oxygen content) depends both on the PO2 (which affects amount dissolved and amount added to Hg) and on the haemoglobin concentration. - In adult males, the normal haemoglobin levels are 13.8 - 17.2 g/dl – note the units involve decilitres (men prob at the higher end, women prob at the lower end; prob dec with age). Decilitre = 100ml - the oxygen content of arterial blood in a healthy person with a Hb concentration of 15 g/dl is around 9mmol/L - If you breathe an oxygen enriched mixture, alveolar PO2 may rise to (say) 55 kPa (4 x higher than normal) the arterial PO2 will scarcely rise at all, since at 13 kPa the Hb is already virtually fully saturated anyway (are at the flat top section of the sigmoidal curve) - Anaemia: alveolar and arterial PCO2 will be pretty normal (unless the anaemia is extremely severe) because it is the pa(CO2) that regulates ventilation and this is not affected by a drop in Hb levels - If the inspired gas was 100% oxygen, all the nitrogen normally present in alveolar air will gradually be replaced by oxygen and, in consequence, alveolar PO2 will increase enormously and eventually rise above 80 kPa but this will not (a) significantly improve and/or (b) correct oxygen delivery to the tissues in an anaemic subject because regardless of Hb conc, saturation has already almost been reached at a p(O2) of 13kPa (the small rise in anaemia and non-anaemia is similar in both cases) (main help is in reducing the ventilation rate required to sustain a p(O2) of 13+kPa – however note that no help will be given in aiding CO2 removal by using high conc O2 to breath; must hope that the ventilation rate stimulated by the pa(CO2) levels will be sufficient) 39 Giles Kisby - - - - - - GE Y1 Respiratory Must know normal values for pA(O2) [13.3kPa], pa(O2) [13kPa] and pa(CO2) [5.3kPa] as is expected knowledge in helping pick a correct answer Alveolar PCO2 in a child aged six is roughly the same as it is in an adult. Haemoglobin is abbreviated Hb or Hgb (not Hg) Hb dissociation curves: The shape of the curve does depend on the PCO2 (as this alters the affinity of Hb for oxygen) but it is independent of the Hb concentration. However, if the actual AMOUNT of oxygen is plotted on the y axis instead of the percentage saturation of Hb, there is a marked change with inc Hb. Must consider what is 'normal' for someone of that AGE and SEX – these factors influence what is a normal result. Having said this, as an approximation, 15g/dl can be taken as a typical value for the Hb concentration in the blood of a healthy adult. The rate of oxygen consumption of the tissue concerned AND its rate of blood flow affects how much O2 is removed from the blood: O2 consumption = O2 delivered – O2 returned Which is … o VO2 = (Q x Ca O2) – (Q x CvO2) o VO2 = Q (CaO2 - CvO2) o VO2/Q = CaO2 –CvO2 o Where Q= rate of blood flow [not same as CO for all tissues but CO used when considering all tissues as a whole], VO2 = O2 consumption, CaO2 = oxygen concentration in arterial blood, CvO2 = oxygen concentration in venous blood. o This is the Fick principle breathing an oxygen enriched mixture where alveolar PO2 is 55 kPa (4 x higher than normal): If the PO2 in alveolar air is 55 kPa and that in the blood is 13 kPa, then there is a concentration gradient across the alveolo-capillary membrane and O2 WILL diffuse into the blood thus raising the concentration of free' O2(i.e. NOT bound to Hb) (ie the P(O2)). N.B. The best way of regarding the PO2 of the blood is as a measure of the amount of 'free' O2. This is not readily saturated -so the PO2 can definitely rise above 13 kPa. HOWEVER the amount of oxygen bound to Hb will scarcely rise at all as the binding sites on the Hb are almost all already 'full' when the PO2 is 13 kPa. Although the amount of 'free' O2 goes up fourfold as the PO2 rises from 13 kPa to 55 kPa, the effect on the overall O2 content of the blood is small - because O2 is poorly soluble. what do you think will happen to arterial PO2 in a moderately anaemic subject at rest: The PO2 of mixed alveolar air depends on (i) the alveolar ventilation (ii) the rate of oxygen consumption and (iii) the composition of inspired gas. None of these will be changed in anaemia. The same ventilation that results in an alveolar PCO2 of 5.3 kPa also results in a PO2 of roughly 13 kPa in a healthy subject. [ie The best way of regarding the PO2 of the blood is as a measure of the amount of 'free' O2 – the Hb is not relevant]. In anaemic subjects alveolar and therefore arterial PO2 will NOT be lower than normal Breathing an oxygen-enriched gas mixture is not very effective at increasing O2 delivery to the tissues in anaemia. although the amount of ‘dissolved O2' will be substantially increased, breathing 100% oxygen (in someone with normal lungs) has little or no effect on the amount of oxygen 40 Giles Kisby - GE Y1 Respiratory bound to Hb. Consequently, there will only be a comparatively small increase in the overall O2 content. Breathing an oxygen-enriched gas mixture will NOT improve the raised PCO2 resulting from hypoventilation. Basically the only factors that determine alveolar PCO2 are (a) the ventilation and (b) CO2 production. There is no obvious reason why either of these will change much when breathing O2 and if they don't, PACO2 will stay unchanged. However, in some patients with Chronic obstructive airways disease (i.e. COPD) who hypoventilate the arterial PO2 may be so low that it helps to drive breathing (ie oxygen level can signal for breathing rate change but normally are at flat part of sigmoidal curve so a lowered RR will usually give a CO2 inc before a O2 dec so CO2 tends to signal first before O2 gets a chance – these patients develop a resistance to high blood CO2). If so then if you improve the PO2 by giving oxygen, this drive to breathe will be removed and the ventilation may fall to even lower levels - which in turn will result in a further (potentially dangerous) RISE in PCO2 and FALL in pH. 41 Giles Kisby GE Y1 Respiratory 14/11/13: Respiratory practicals 1&2: 1. Los (from sheet): 1. Describe spirometry procedures to measure lung volumes and capacities. 2. State approximate values for lung volumes in a young healthy adult. 3. Appreciate how body height, weight, age and gender influence lung volumes, and can be used to predict these values. 4. List lung volumes/capacities that can be measured by simple spirometry. 5. Be aware of how these volumes may change during exercise. 6. Identify lung volumes/capacities (including: RV, FRC, VC, TLC) that are affected by: (1) severe chronic restrictive lung disorder (2) severe chronic obstructive pulmonary disorder, and be able to give reasons for these changes. Notes: - The volumes of air present in the lungs/airways are described in terms of “Volumes” and “Capacities” (a capacity is made up of 2 or more volumes): - The standard lung volumes are: o Tidal Volume (VT or TV). The volume of air inspired (or expired) in a single “spontaneous” breath. o Inspiratory Reserve Volume (IRV). The additional volume (i.e. in reserve) of air that could be inspired at the end of a VT inspiration. o Expiratory Reserve Volume (ERV). The additional volume (i.e. in reserve) of air that could be expired at the end of a VT expiration. o Vital Capacity (VC). The maximum volume of air that it is possible to exhale from the lungs following a maximal inspiration. (VC = IRV + VT + ERV). o Inspiratory Capacity (IC) The volume of air that it is possible to inspire at the end of a normal quiet expiration. (IC = VT + IRV). o Residual Volume (RV). The volume of air remaining within the lungs/airways at the end of a maximal expiration. (Maximum Expiratory Level in diagram) o Functional Residual Capacity (FRC). The volume of air contained within the lungs/airways at the end of a quiet VT expiration (Resting Expiratory Level in diagram). This is the equilibrium volume at which the elastic recoil forces of the lungs pulling inwards exactly balance the forces pulling the chest wall structures outwards. (FRC = RV + ERV) o Total Lung Capacity (TLC). The volume of air contained within the lungs/airways at the end of a maximal inspiration. (Maximum Inspiratory Level in diagram). (TLC = RV+ERV+VT+IRV). 42 Giles Kisby - - - - - GE Y1 Respiratory [Note: Normal values for lung volumes depend on gender (lower for female), age (decrease with age) and body size (lower if small)] o Equations factoring in these variables can be used to gain predicted volume values for that person Normal values in young healthy adults (20-30 years): o Costanzo figures: IR=3L, TV=0.5ml, ER = 1.2L, RV = 1.2L, VC = 4.7L, TLC = 5.9L, FRC = 2.4L, IC = 3.5L o Female: Vital capacity: 3.7L Residual volume: 1L o Male: Vital capacity: 4.6L Residual volume: 1.2L Measurement of lung volumes with simple spirometry: o The lung volumes measured are made without having to breathe out as fast as possible; the respiratory movements can be carried out slowly. o While seated comfortably and wearing a nose-clip, breathe from the spirometer for 5 normal breaths, (VT) followed by a maximum inspiration, hold for 1-2 seconds; then a slow maximum expiration, hold for few seconds; then breathe normally again for 2 normal breaths. o Results from the machine are: VT (litres), IRV (litres), ERV (litres), VC (litres), IC (litres) [ie everything but FRV] but the volumes measured from a spirometer trace are in ATPS (i.e. for the air at atmospheric temperature & pressure & saturated) and so underestimate the volume actually occupied by that amount of gas – have to use a constant to change to BTPS (body temperature & pressure & saturated) Restrictive lung disorders o VC: Restrictive lung disorders are characterised by an abnormally low thoracic “compliance”. In consequence, the ability of the patient to expand the lungs is “restricted”. An example of such a disorder is interstitial pulmonary fibrosis, in which (as its name implies) there is a substantial increase in the amount of collagen contained within the pulmonary interstitium. Ie reduced VC due to reduced IRV Obstructive lung disorders o RESISTANCE: In obstructive lung disorders, some or all of the airways are “obstructed” i.e. narrowed. The most common lung diseases including asthma and chronic obstructive pulmonary disease (= chronic obstructive airways disease + bronchitis + emphysema) have a large obstructive component (inflammation, airway trapping, hypersecretion of mucus, etc). In considering this increase in resistance, FEV1 / VC is prob a good measure of this; should be >80%. o VC: VC is often reduced in patients suffering from chronic obstructive pulmonary disorder: this is largely due to loss of elastic fibres for preventing overinflation of 43 Giles Kisby GE Y1 Respiratory alveoli and for aiding alveoli contraction in exhale therefore lungs are more sensitive to the process of airway trapping (collapse of bronchioles at specific positions) and less able to evacuate airways so giving reduced VC via reduced ERV. [also, airway trapping occurs and may contribute to reduced ERV] Ie reduced VC due to reduced ERV 2. Los (from sheet): 1. Briefly describe two indirect methods to evaluate airways resistance. 2. Define FVC, FEV1, and PEFR 3. Explain why FEV1 is reduced in obstructive and in restrictive lung disease. 4. Explain the significance of the ratio FEV1/FVC and state its normal value. 5. Explain why the Wright Peak Flow meter is particularly useful for patients with asthma or COPD. 6. State that values for FVC, FEV1, and PEFR are generally lower in females, increase with subject’s height and decrease with age peaking at 20 years. Notes: - - - - Many (obstructive) respiratory disorders result in a reduction of the diameter of the airway lumen and hence an increase in the resistance to flow making breathing more difficult. Indicators of airways resistance can provide useful information on the severity and progression of respiratory disease and on the efficacy of any treatment. Airways resistance is the pressure difference between alveoli and mouth divided by the rate of air flow. (ie from the “Flow = ΔP / R” equation) Measurement of this pressure difference is technically tricky and this is not routinely done for testing a patient’s respiratory function. However, if the airways are narrowed by disease, then flow rates during forced expiration will be lower than expected. Ie ΔP exerted prob constant but with obstructive disease will achieve lower flow (FEV1 / VC is prob a good measure of this; should be >80%) The values obtained will depend upon the age, size and gender of the subject, but if expressed as a % of the predicted value for that individual they provide a simpler, indirect indication of airways resistance. The following forced expiratory measurements can be made: o Forced expiratory volume in 1 second (FEV1): This is the total amount of air in litres that you can forcibly blow out in one second after full inspiration. Measured by a vitalograph o Forced vital capacity (FVC): This is the total amount of air in litres that you can forcibly blow out after full inspiration. Measured by a vitalograph [Vital capacity can be measured as forced vital capacity (FVC), slow vital capacity (SVC; prob the same as above but slow), and inspiratory vital 44 Giles Kisby - - - GE Y1 Respiratory capacity (IVC; prob means working from full expiration to full inspiration as the measure). Note it is well known that the latter two are generally greater] o Peak expiratory flow rate (PEFR): This is the highest speed at which the air moves out of your lungs at the beginning of the forced expiration, measured in litres per second. Measured by a Wright peak flow meter Using a vitalograph: o Adopt an upright position – standing erect. o Ensure vitalograph is primed and reset to the starting position o First inspire maximally, and then seal your lips around mouthpiece o Blow as hard and fast and as far as possible into vitalograph o Try not to come off to breathe in again until vitalograph reaches the end o FEV1 and FVC are read off the trace (trace already calibrated to give readings in BTPS) Using a Wright peak flow meter: o Adopt an upright position – standing erect. o Ensure meter is zeroed o Seal your lips around mouthpiece with neck slightly extended o Blow as hard and fast as possible applying maximal effort. o PEFR is determined What can FVC, FEV1 and FEV1/FVC tell us about lung pathology? o 1) An individual with an obstructive respiratory disorder (such as asthma or COPD) will have impaired ability to exhale quickly because of obstruction to air flow. A reduced airway caliber can occur due to: bronchial oedema excess mucus secretion smooth muscle hypertrophy bronchospasm (constriction of the muscles in the walls of the bronchioles: think asthma) inflammation (think bronchitis) floppy airways (think emphysema) airflow trapping (think emphysema) o A low FEV1 (due to the above obstructions) and a low FVC (due to some complete airway trapping) is the norm in these obstructive pathologies. The severity of obstruction is determined by the FEV1/FVC ratio: An FEV1/FVC ratio < 75% = indicates mild air flow limitation An FEV1/FVC ratio < 60% = indicates moderate air flow limitation An FEV1/FVC ratio < 40% = indicates severe air flow limitation o 2) An individual with a restrictive respiratory disorder (pulmonary fibrosis or a neuromuscular condition preventing “normal” lung inflation) will normally have low FVC (due to low ERV). Unless there is a second pathology limiting airflow, FEV1 will be proportionately normal (ie both FEV1 & FVC reduced): “FEV1 is 45 Giles Kisby GE Y1 Respiratory o o reduced in both obstructive and restrictive lung disease”]. So in restrictive disease, although the FVC is low, The FEV1/FVC ratio will be ≥ 75% In certain restrictive respiratory disorders, the FEV1, and FEV1/FVC ratio may be higher than normal. The reason for this relates to how the expiratory flow is generated. In normal compliant lungs, expiration depends on elastic recoil of the alveoli producing a measured expiratory flow. In restrictive disorders due to pulmonary fibrosis, collagen fibres replace elastic fibres, the alveoli and bronchioles become stiffer than normal, and normal elastic recoil is lost. Loss of elastic recoil tends to produce alveoli that aggressively recoil on expiration producing a high FEV1. Thus expiration may occur even faster than normal. In any condition in which FEV1 is preserved or enhanced and where FVC is simultaneously lower than normal, the resultant FEV1/FVC will always be higher than normal i.e. > 75%. Overall, you can think of restrictive conditions as being disorders in which lung expansion is impaired, but the ability of the lung to empty is preserved (or enhanced). - Vitalograph traces: Left one is for restrictive lung disease and right graph is for obstructive lung disease: - How is measurement of PEFR used clinically? o A measure of the highest flow rate achieved during a forced expiration after a full inspiration indicates the ease with which expiration occurs. The chief limitation of this test is that it only reflects resistance to airflow in larger, more central airways. Clinically, the peak expiratory flow meter has 2 main uses; to help confirm or refute a possible diagnosis, and to provide a convenient way to monitor a patient’s progress, deterioration or response to therapy. - Peak expiratory flow rate (PEFR) data: o An adult male (20-50 years) without lung pathology should score between 540 and 670 l.min-1. (see graph) 46 Giles Kisby GE Y1 Respiratory o o o o o - An adult female (20-50 years) without lung pathology should score between 400 and 470 l.min-1. (see graph) Since the test is effort dependent, results will vary, depending on the effort one puts in. To make an individual score meaningful it is common practice to refer to normative data categorised according to gender age and build etc. An adult with obstructive disease will score significantly lower than an individual with healthy lungs. With patients it is important to note intra-subject variability (should always repeat) How might you classify the following conditions? Restrictive or obstructive? Remember to ask yourself if the condition; a) limits the ability of the lung to fill, b) limits the ability of the lung to empty: - - Asthma: obstructive COPD [Chronic obstructive pulmonary disease (COPD) is the name for a collection of lung diseases including chronic bronchitis, emphysema and chronic obstructive airways disease]: obstructive Bronchiectasis: obstructive [Involved bronchi are dilated, inflamed, and easily collapsible, resulting in airway obstruction and impaired clearance of secretions] cystic fibrosis: obstructive [It is characterized by abnormal transport of chloride and sodium across an epithelium, leading to thick, viscous secretions] 47 Giles Kisby GE Y1 Respiratory - pulmonary fibrosis: Restrictive non-productive pneumonia: restrictive (ie if was producing fluid further up the airways then could have an obstructive component too) Pneumothorax: restrictive Kyphoscoliosis: restrictive (curvature of the spine) Neurological and neuromuscular disorders resulting in difficulties activating respiratory muscles [like Guillain–Barre, motor neurone disease, multiple sclerosis, polio, or myasthenia gravis]: restrictive 48 Giles Kisby GE Y1 Respiratory 19/11/13: Regulation of Breathing (Awake & Asleep) Los (from slides): • • • • • • • • • • • • • [says we must understand blood gases (and know their normal values) and be able to interpret lung function (ie as well as the below)] Distinguish the primary purpose of the automatic reflex and the behavioural controller Define neuronal groups in the brainstem that make up the automatic reflex controller for breathing, and structures in higher brain areas (suprapontine) that drive behavioural (non-automatic) control of breathing. Explain how they can act independently or interact for control of the respiratory pump. Recall the sources of sensory input to the respiratory control system and the common motor outputs (also see Lecture 11) Explain the ventilatory response to increased arterial PCO2, decreased arterial PO2 Recognise breathlessness (“dyspnoea”) and start to consider its role in breathing control (lectures 12,13&15). Recognise the effects on respiratory control of the neurological conditions; ‘locked in’ syndrome and ‘congenital central hypoventilation syndrome’ Explain the effect sleep on breathing and blood gases in healthy people Summarise the changes in chemosensitvity that occur during sleep and define the apnoeic threshold Explain how the changes in chemosensitivity and the apnoeic threshold led to central sleep apnoea Explain the influences of sleep on the upper airway which lead to obstructive sleep apnoea Recall one major cardiac, one major respiratory disease that is exacerbated by the sleeprelated changes in the control of breathing Notes: - Key aspects of breathing control: o To regulate gas exchange 1. for homeostasis: Acid base balance 2. for metabolism: At rest O2 consumption ~ 250 ml.min-1; At rest alveolar ventilation ~ 5 l.min-1. Exercising O2 consumption can increase by 20 fold; alveolar ventilation must increase to match this demand. o To execute behavioural acts communication speech, singing, playing wind instruments emotion laughter, crying, anxiety 49 Giles Kisby GE Y1 Respiratory o - non respiratory functions swallowing defecation, micturation, parturition (giving birth), vomiting To maintain airways and lung function Coughing, sneezing, yawning, sighing Control the pharynx and larynx to maintain upper airways patency (note that there is no cartilage in the uppermost area of the airways) [Pharyngeal muscle activity decrease during sleep contributes to obstructive sleep apnoea] Control the pump muscles for inspiration and for expiration Inputs to respiratory muscle control: o Voluntary/behavioural Ie are able to voluntarily change our blood gases up to a point Motor cortex (from the part of the cortex between the “shoulder” and “trunk” regions (ie makes sense for it to be there) Outflow of signals is via the corticospinal pathway to the cervical chord before distribution to the diaphragm o Emotional Limbic system Poorly understood but is known to exist due to studies on patients with locked in syndrome showing changes to breathing rhythm in response to emotion (locked in patients have a lesion above the bulbospinal tract and so while autonomic breathing is unaffected and all sensory inputs are present there is no voluntary muscle control (except for eyes)) o Reflex/autonomic Brainstem (mainly the medulla but the pons has some role too) Pacemaker for respiratory rhythm generation is thought to be present in the Pre-Bötzinger Complex (preBötC) of the medulla [the pacemaker is understood to be the result of interconnections of the different respiratory neurones (early inspiratory neurones + late inspiratory neurones + expiratory neurones) which interact by reciprocally inhibiting each other (to result in the observed rhythm)] Outflow of signals: Outflow of signals to the pump muscles (Diaphragm, Intercostal Muscles, Abdominal muscles) is via the bulbospinal pathway / tract to the cervical chord before distribution to the “pump muscles”: o Diaphragm phrenic nerve; cervical plexus (C3 - C5) o Intercostal Muscles T1 - T12 o Abdominal muscles 50 Giles Kisby GE Y1 Respiratory T12, L1 Outflow of signals to the upper airway muscles is predominantly via cranial nerves o glossopharyngeal (IX) o vagus (X) o spinal accessory (XI) o hypoglossal (XII) Autonomic regulation of breathing takes its input from changes to ppCO2, pH and ppO2 (though will also take inputs from respiratory muscles and lung inflation so is “aware” of what results it is producing – see below diagram) Sensory inputs for exercise: cortical, ergoreceptors, cardiodynamic (as well as all of the below) Sensory inputs: [nb J-receptors, irritant, stretch, muscles/joints] o Nose Trigeminal (V) o Pharynx Glossopharyngeal (IX) Vagus (X) o Larynx Vagus (X) o Lungs Vagus (X) o Chest wall spinal nerves [Chemoreceptor – type sensory inputs]: o Peripheral chemoreceptors carotid bodies, aortic arch hypoxia (ie mainly involved in O2 sensing), (also some pH, PCO2 sensing but this is mainly done by the central chemoreceptors) o Central chemoreceptors [presumably these are the ones important in regulating ventilation as they are key in CO2 detection] located on the surface of the medulla and detect changes in CSF PCO2 , pH (ie NO O2 SENSING) 51 Giles Kisby GE Y1 Respiratory [note: only autonomic and voluntary pathways are annotated as little known about mechanism of emotion input] - Disorders of breathing during Sleep: o Central sleep apnoea: Gives Cheyne–Stokes respiration / Cheyne–Stokes breathing. This is a type of breathing in which ribcage/thoracic effort and abdominal effort (as plotted as displacement of that structure anterior / posterior) shows a period of baseline / zero effort followed by crescendodecrescendo effort before the cycle repeats In effect is repeated over and under breathing in an attempt to find an optimum and is associated with falling asleep with an abnormally low blood CO2 concentration Can occur if woken up and then fall back to sleep (while asleep a high blood CO2 conc is tolerated but upon waking this needs to be lowered and high respiratory rate is stimulated; this can overcompensate to give abnormally low blood CO2 concentration and therefore if then fall asleep will give a starting point for the repeated over and under breathing in an attempt to find an optimum Is also associated with heart failure patients because they will likely suffer from pulmonary congestion so tend to overbreathe in response (ie in fact therefore tend to have lower than usual 52 Giles Kisby GE Y1 Respiratory o blood CO2 concentration) so if fall asleep the conditions for encouraging Cheyne–Stokes breathing are met A final group at risk are babies with Ondine's curse = CCHS (congenital central hypoventilation syndrome): a congenital or trauma induced brain problem Treatment is artificial ventilation during night Obstructive sleep Apnoea Involves collapse of the pharyngeal region of the airway; no cartilage at this region so in obese patients with big necks collapse can occur during inspiration when the negative pressure adds to the weight of the fat to overcome the patency. [normally maintained by neural tone of this part of the airway but Pharyngeal muscle activity decreases during sleep] Obstruction causes paradoxical breathing (as mimicked by closing mouth and pinching nose) which is where inspiration gives posterior movement of abdomen and anterior movement of thorax and visa versa for expiration (occurs to far less a degree when airways patent) Gives cycles (see below) like central sleep apnoea but this is just because patient keeps waking up and immediately falling back to sleep (prob won’t realise they have woken up) Treated with a mask to blow air in during inspiration [see below as the restriction at back of tongue which will seal during sleep inspiration]: ***Importantly central sleep apnoea is no airflow and no effort whereas obstructive sleep apnoea has no airflow but effort occurs 53 Giles Kisby GE Y1 Respiratory - Ventilatory sensitivity slopes: o Refers to the first of the two graphs below; is investigating sensitivity of ventilation rate to an increasing concentration of inhaled CO2 (subject effectively breathes into bag so CO2 conc increases) o Varies between subjects, some people naturally more sensitive than others; for all subjects though the sensitivity drops during sleep (ie reduced gradient) as tolerance to elevated CO2 levels increases (a higher benchmark for CO2 conc becomes established) o The second graph is just to show that ventilation remains constant for a wide range of PAO2 values (horiz flat line region) and so is not having an input into ventilation redulation (except possibly at very very extreme O2 levels) - During sleep only autonomic control of breathing is occurring (not voluntary / emotional) During waking state an EEG shows low amplitude, high frequency signals, During sleep EEG shows High amplitude, Low frequency signals – except for REM sleep which shows trace similar/same to waking state (breathing is still different though because during sleep only autonomic control of breathing is occurring) Sleep cycle is ~90mins with 1x REM per cycle and length of REM in cycle lengthens with each cycle Antidepressants supress REM During sleep the respiratory volume falls (but the frequency actually doesn’t change) so the minute ventilation and alveolar ventilation fall by ~10%: o CO2 levels in blood will therefore rise but due to increased tolerance of blood CO2 (less sensitive chemoreceptors) a new baseline will be reached (consistent with the respiratory volume fall). o In turn the pa(O2) of blood will fall but this is not a problem in most people because we are at the flat part of the oxygen dissociation curve therefore the O2 saturation of tissues will not be affected (see graph below) o However if the patient has lung disease (eg COPD) then due to their exchange problems they will already be near the edge of the flat part and so there will be a fall into the more vertical region of the plot and problems relating to - - 54 Giles Kisby GE Y1 Respiratory saturation will arise (tissues not getting enough blood) [nb their CO2 sensitivity will also likely be lower already (due to disease induced bradypnoea, loss of alveoli, and obstruction including hypoxic vasoconstriction) and breathing may be partially O2 driven so inc inc in CO2 and fall of HB sats may not be as big as otherwise] Excel monster notes extras: - Resting position of chest wall 70-80% of TLC Resting position of lung ~0% TLC capnic (CO2 driven) Normal resting figures [APPROXIMATION – is variable]: O2 consumption = 200-300 ml/min, CO2 production = 200-250 ml/min Amount of oxygen bound to haemoglobin is 20mls/100mls of blood so 1g haemoglobin binds up to 1.34mls of O2 for Hb normal at 15g/dl (ie 100ml = 1dl) Also: the oxygen content of arterial blood in a healthy person with a Hb concentration of 15 g/dl is around 9mmol/L During inspiration the chest wall is expanded and the intrapleural pressure falls. This increases the pressure gradient between the intrapleural space and the alveolar pressure, stretching the lungs. The alveoli expand and and alveolar pressure falls creating a pressure gradient between the mouth and alveoli causing air to flow into the lung. The airflow profile closely follows that of alveolar pressure. During expiration both intrapleural pressure and alveolar pressure rise. In quite breathing, intrapleural pressure remains negative for the whole of the respiratory cycle, whereas alveolar pressure is negative during inspiration and positive during expiration. Alveolar pressure 55 Giles Kisby - - - GE Y1 Respiratory is always higher than intrapleural pressure, because of recoil of lung it is zero at the end of both inspiration and expiration and airflow ceases momentarily. When ventilation is increase the changes of intrapleural and alveolar pressure are greater than and in expiration intrapleural pressure may rise above atmospheric. In forced expiration, coughing or sneezing, intrapleural pressure may rise to +8kPa or more. Oxygen dissociation haemoglobin curve o to the left: increase pH, decrease oCO2, decrease temperature decrease 2,3,DPG [2,3-diphosphoglycerate; metabolic product present in respiring tissues] o To the right: decrease pH increase co2 increase temperature increase 2,3 DPG Half of airway resistance lies in the nose, pharynx and larynx (prob is referring to start of exhale) Anatomical dead space 30% of tidal volume During REM patients with COPD cannot use accessory muscles for breathing (if are essential then will start to suffocate and will wake up) Efficiency of gas exchange = Pulmonary venous PO2/ alveolar P02 [usually is very high] Vascular resistance = (arterial pressure - venous pressure) / cardiac output The alveolar partial pressure of oxygen PAO2 can be calculated from the following equation: PAO2 = PIO2 – (PaCO2/R) o R is the respiratory quotient, which represents the amount of carbon dioxide excreted for the amount of oxygen utilized, and this in turn depends on the carbon content of food (carbohydrates high, fat low) o PIO2: the partial pressure of inspired oxygen 1 kPa = 7.5 mmHg (for converting purposes) 56 Giles Kisby GE Y1 Respiratory [Exact normal figures depend on age, height, weight and gender but these figures (from the notebank) match closely with the excel notes so are good general estimates (prob are really for male; bit lower for female) and worth learning] 57 Giles Kisby GE Y1 Respiratory 21/11/13: Sensory Aspects of Respiratory Disease: Los (from slides): Prof. Fan Chung • • • • General • Understand how respiratory symptoms are generated and perceived • Discuss the importance of measuring respiratory symptoms in clinical medicine and clinical research • Outline the clinical causes and pathophysiological basis of the respiratory symptoms cough, chest pain (and dyspnoea, covered elsewhere): Cough • Describe the mechanics of a cough with reference to inspiration, expiration and closure of the glottis. Briefly explain how this manouevre serves to i) protect the lungs from inhaled noxious materials and ii) clear excessive secretions from the lower respiratory tract • Identify the type and location of sensory receptor within the airways indicating how these are stimulated to give rise to cough. Identify the neural pathways which transmit this afferent (sensory) information to the brain • Describe which regions of the brain are involving in generating the co-ordinated neural activity that results in a cough. Identify the efferent (motor) neural pathways and the main muscle groups which produce cough. • Explain the concept of the sensitised cough reflex in disease as a basis for chronic cough. • Discuss ways of controlling unnecessary cough Chest pain • Identify the type and location of sensory receptors within the thoracic cavity that when stimulated give rise to chest pain. Identify the neural pathways that transmit this afferent neural information to the brain. • Describe in outline which regions of the brain are involved in the perception of pain • Discuss the concept of referred pain in the chest • Describe typical patterns of chest pain that can help in diagnosing the cause of pain Dyspnoea • Review the terms used by patients to describe the troublesome symptom of shortness of breath and its measurement • Discuss the main important causes of shortness of breath and approach to management Notes: - Recommended textbook: Murray & Nadel’s Textbook of Respiratory Medicine 5th Edition 2010 Volume 1 o Chapter 28. Dyspnea o Chapter 29. Cough o Chapter 30. Chest pain - Prevalence/importance of respiratory symptoms o Cough Third most common complaint heard by GP o Chest pain Most common pain for which patient seeks medical attention o Shortness of breath (SOB, dyspnea) 58 Giles Kisby - - GE Y1 Respiratory 3% of visits to A&E Pathway of physiologic or pathological stimulus leading to conscious sensation: 1. Sensory stimulus 2. Transduction of the signal to the nerve by receptors 3. Excitation of Sensory nerve 4. Integration of signals at CNS 5. Sensory impression 6. Perception 7. Evoked sensation Cough: o A crucial defence mechanism protecting the lower respiratory tract from: inhaled foreign material excessive mucous secretion o Usually secondary to mucociliary clearance (cilia move debris to the larger airways and then either completes removal to esophagus or cough occurs) but important in lung disease when mucociliary function is impaired and mucous production is increased (ie here cough becomes more important) o Expulsive phase of cough generates a high velocity of airflow facilitated by bronchoconstriction and mucous secretion. o Localisation of cough receptors Rapidly adapting irritant receptors which are located within airway epithelium. Most numerous on posterior wall of trachea, Also: at main carina, and branching points of large airways, less numerous in more distal airways. Absent beyond the respiratory bronchioles [ie beyond the first main bronchi] Also in the pharynx. Possibly also in the external auditory meatus, eardrums, paranasal sinuses, pharynx, diaphragm, pleura, pericardium, and stomach. Stimuli: laryngeal and tracheobronchial receptors respond to chemical and mechanical stimuli. 59 Giles Kisby GE Y1 Respiratory o Sensory receptors in the lung and airways Slowly adapting stretch receptors [Fibers that respond to movement and also static indentation are termed slowly adapting mechanoreceptors; respond to stretch but also produce sustained responses to static stimulation] Rapidly adapting stretch receptors [Fibers that respond only to movement are termed rapidly adapting mechanoreceptors. Underlies the perception of flutter and slip. Produce transient responses to the onset and offset of stimulation] Come under the category of “A fibres” Location: Naso-pharynx, larynx, trachea, bronchi Small, myelinated nerve fibres Because of their higher conduction velocity (compared to C fibres), Aδ fibers are responsible for the sensation of a quick shallow pain that is specific on one area, termed as first pain. They respond to a weaker intensity of stimulus Stimuli: Mechanical, chemical irritant stimuli, inflammatory mediators C – fibre receptors Location: Larynx, trachea, bronchi, lungs Small, unmyelinated fibres [C fibers are unmyelinated unlike most other fibers in the nervous system] C fibers respond to stimuli which have stronger intensities and are the ones to account for the slow, but deeper and spread out over an unspecific area, second pain “free” nerve endings: [C fibers and the majority of Aδ fibers end as free nerve endings - an unspecialized, afferent nerve ending, meaning it brings information from the body's periphery toward the brain] Stimuli: Chemical irritant stimuli, inflammatory mediators 60 Giles Kisby GE Y1 Respiratory o Release neuropeptide inflammatory mediators Substance P, Neurokinin A, Calcitonin Gene Related peptide In addition other cough receptors are thought to be present as determined from staining studies, etc and are not covered in the above categories Afferent neural pathways for cough; Stimulation may be mechanical (e.g. dust, mucous, food/drink) Stimulation may be chemical (e.g. noxious, intrinsic inflammatory agents) From the positions of the sensory receptors the signals are transmitted via the vagus (or by the superior laryngeal nerve (a branch of the vagus) in the case of receptors in the pharynx which then join the vagus) Vagus takes signals to the “cough center” at the medulla; some signalling is also passed on to the cerebral cortex (ie o some voluntary control of coughing can occur eg in suppression of coughing) Efferent neural pathways for cough Signal flow out from the “cough center” at the medulla; and some signalling occurs from the cerebral cortex (ie some voluntary control of coughing can occur eg in suppression of coughing) Signals flow to a complete range of the muscles involved in breathing; both inspiratory and expiratory because cough involves first a intake of breath before it is then expelled The Glottis is also the recipient of signals and during the cough will remain closed allowing the subglottic pressure to rise before then allowing the forceful release of air; this should clear the airways and is responsible for the first, loud, cough sound Following this there is a second, quieter, cough sound generated due to the vibration of the glottis fold as the pressures are re-established 61 Giles Kisby - - GE Y1 Respiratory Sensitising of the afferent pathways is possible and is thought to be underlying many diseases: they often involve cough reflexes to stimuli that would not cause stimulation in a healthy subject [Cough is typically difficult to control (ie is not throat clearing)] o Excitability of afferent nerves is increased by chemical mediators eg prostaglandin E2 o Increase in receptor numbers seen o Neurotransmitter increase eg neurokinins in brain stem Causes of cough: o Acute infections o Chronic infections o Airway diseases Asthma Chronic bronchitis o Parenchymal disease Interstitial fibrosis Emphysema o Tumours o Foreign body o Cardiovascular Left ventricular failure Pulmonary infarction Aortic aneurysm o Reflux oesophagitis o Drugs Angiotensin converting enzyme o Acute cough is <3 wks o Chronic cough is >3 wks Asthma and eosinophilic-associated (25%) Gastro-oesophageal reflux (25%) Rhinosinusitis (postnasal drip) (20%) Chronic bronchitis (‘smoker’s cough) (8%) 62 Giles Kisby GE Y1 Respiratory o o Bronchiectasis (5%) Drugs eg Angiotensin converting enzyme inhibitor (1%) Post-viral (3%) ‘Idiopathic’ (10%) Other causes (3%) Complications of cough: Pneumothorax with subcutaneous emphysema Loss of conciousness (cough syncope) Cardiac dysrythmias Headaches Intercostal muscle pain Rupture of rectus abdominis juscle Social embarrasment Depression Urinary incontinence Wound dehiscence Cough treatments: - Antitussive: Capable of relieving or suppressing coughing. o Eg opiates - Chest pain: o Sensory input from lungs, airways and chest wall: Nose Trigeminal (V) Pharynx Glossopharyngeal (IX) Vagus (X) Larynx Vagus (X) Lungs Vagus (X Chest wall spinal nerves o Touch sensation vs pain sensation: 63 Giles Kisby GE Y1 Respiratory Different receptors: touch = A, A; pain = A, C-fibres Differ at level that the fibres change to the contralateral side (pain crosses at the same level that access to the spine occurs whereas touch fibres cross higher up in the lower brain region) Ultimately both travel to the thalamus and some onto the cortex o o o o o o [output will be perception in brain and any relevant outflow signalling to respond] Different types of pain: somatic vs visceral Visceral pain (from visceral organs eg heart, gi tract, bronchial wall) is not the same as somatic pain (from skin). Visceral pain is difficult to localise, diffuse in character and is referred to somatic structures. Number of visceral afferents is less than number of somatic afferents Pain arising from various viscera in the thoracic cavity and from the chest wall is often qualitatively similar and exhibits overlapping patterns of referral, localisation and quality, leading to difficulties in diagnosis. Chest pain from respiratory system: Pleuropulmonary disorders: Pleural inflammation eg infection, pulmonary embolism, Pneumothorax, malignancy eg mesothelioma Tracheobronchitis: Infections, inhalation of irritants Inflammation or trauma to chest wall: Rib fracture, Muscle injury, Malignancy, Herpes zoster (intercostal Nerve pain) Referred pain: shoulder-tip pain of diaphragmatic irritation Chest pain from‘non-respiratory’ disorders: Cardiovascular disorders Myocardial ischaemia/infarction Pericarditis Dissecting aneurysm Aortic valve disease Gastrointestinal disorders Oesophageal rupture Gastrooesophageal reflux Cholecystitis Pancreatitis ‘Psychiatric disorders’ panic disorder Self-inflicted PET is an important tool in pain studies; active areas show up on scan Many different brain areas are activated during pain: 64 Giles Kisby GE Y1 Respiratory o - Somatosensory processing: Motor processing Affective processing: Attentional processing: Autonomic function; Treatment of chest pain: Treat the cause Chronic pain is more difficult to manage Analgesia may reduce symptoms Pain can be severe & refractory Such cases best dealt with at specialist “pain clinics” Dyspnoea: o General: Troublesome shortness of breath reported by a patient Occurs at inappropriately low levels of exertion, and limits exercise tolerance Can be associated with feelings of impending suffocation. Unpleasant and frightening experience. [same brain areas that are associated with pain are associated with cough therefore explaining the unpleasantness] Poor perception of respiratory symptoms and dyspnea may be lifethreatening A scale of severity is used Exercise testing can be performed o disorders presenting with chronic SOB (dyspnea): Impaired pulmonary function Airflow obstruction eg Asthma, COPD, tracheal stenosis Restriction of lung mechanics eg idiopathic pulmonary fibrosis Extrathoracic pulmonary restriction eg Kyphoscoliosis, pleural effusion Neuromuscular weakness eg Phrenic nerve paralysis Gas exchange abnormalities eg Right to left shunts Impaired cardiovascular function Myocardial disease leading to heart failure Valvular disease Pericardial disease Pulmonary vascular disease Congenital vascular disease Altered central ventilatory drive or perception Systemic or metabolic disease / Metabolic acidosis Anaemia o Treatment of dyspnoea: Treat the cause (eg lung or cardiac) Treatment of dyspnea itself is difficult 65 Giles Kisby GE Y1 Respiratory Therapeutic options: Add bronchodilators eg anticholinergics or b-adrenergic agonists Drugs affecting brain eg morphine, diazepam Lung resection (eg lung volume reduction surgery) Pulmonary rehabilitation (improve general fitness, general health, psychological well-being) 66 Giles Kisby GE Y1 Respiratory 22/11/13: Blood Gases: (someone stood in for Dr Shovlin) Los (from booklet): • Understand what blood gases are, and how they are measured or calculated [this LO is not present on the slide LOs] • Describe the qualitative changes in arterial blood pH, PCO2 bicarbonate and Base Excess in the following acid-base disturbances: o Acute respiratory acidosis o Acute respiratory alkalosis • For (i) and (ii) above, describe the qualitative changes in arterial blood pH, PCO2 and Base Excess following renal compensation. • Describe the qualitative changes in arterial blood pH. PCO2 and Base Excess in the following acid-base disturbances: o Metabolic acidosis with respiratory compensation o Metabolic alkalosis with respiratory compensation • Comment on the mechanism whereby metabolic changes in acid-base status lead to alteration in ventilation and hence respiratory compensation. • Describe the qualitative changes in arterial blood pH. PCO2, Base Excess and PO2 in a patient with (i) Type I respiratory failure (ii) Type II respiratory failure, in each case after full renal compensation. Notes: - Arterial blood gases: o Gives info on: Oxygenation level Acid / base status o Obtained by: Two fingers on radial artery inch apart Ensure pulse on other side of wrist too otherwise hand goes ischaemic Warfarinised needle & syringe takes sample Pressure then used to stop the bleeding (is pulsatile) Sample processed by blood gas analyser o 3x electrodes: “pO2 electrode”: Ag (oxidised) and Pt (reduced) electrodes are involved Careful control of pressure essential as this also affects the current so would give different pO2 reading 67 Giles Kisby GE Y1 Respiratory o o “pCO2 electrode”: [really is referring to a pair of electrodes for pCO2 measurement] Involves measuring the change in pH to a buffer as the CO2 diffuses into it across a permeable membrane “pH electrode”: Involves measuring change in voltage/PD across a membrane (sample one side, buffer the other); will vary depending on the amount of H+ (ie the pH) of the sample Normal values: [these stats should override any from elsewhere] paO2: >10.7kPa [>80mmHg] hypoxic / hypoxia / hypoxaemnia paCO2: 4.7 – 6kPa [35-45 mmHg] Hypercapnia / hypocapnia Hypocapnic / hypercapnic pH: 7.37-7.45 [ie little variation in normal subjects] acidotic / academia / acidosis alkylotic / alkalemia / alkalosis [Hb: 13.3 – 17.7 g/dl] 13.3-17.7 g/dL in adult males and 11.5-16.5 g/dL in adult, nonpregnant females Respiratory influence of pH: Is via a shift in the equilibrium of the below equation: Ie respiratory influence is via paCO2 changes; nb deviations from normal of paCO2 are only from ventilation and respiration influences (though kidney can contribute to resetting CO2 level – see below) In fact there is an intermediate compound that exists: carbonic acid (H2CO3); the full reaction is shown on a diagram lower down Additionally the CO2 can give a decrease in pH by reacting with Hb (haemoglobin) [ie instead of H2O] to form H+: Involves forming a carbamino-CO2 group on the Hb The reverse reaction occurs at the lungs to release the CO2 However, Hb also acts as a buffer by accepting H+ thereby dampening any drop in pH from any cause Ie Hb- +H+ HHb Diagram summarising the reactions: Circulating hydrogen ions and bicarbonate are shifted through the carbonic acid (H2CO3) intermediate to make more CO2 via the enzyme carbonic anhydrase according to the following reaction: 68 Giles Kisby GE Y1 Respiratory o Metabolic influence of pH: Kidney can help regulate pH by regulated excretion of bicarbonate; ie bicarbonate levels will affect the equilibrium of the below reaction: o Kidney regenerates its supply of bicarbonate ions in conjunction with the excretion of H+ ions Metabolic acids can be generated: Eg lactic acid from anaerobic respiration Eg Ketoacids (ketoacidosis) [ketones are formed by the breakdown of fatty acids and the deamination of amino acids; ketoacidosis occurs when the liver breaks down fat and proteins in response to a perceived need for respiratory substrate] o Diabetic ketoacidosis: Insulin stops the use of fat as an energy source by inhibiting the release of glucagon so ketoacids are high in cases of insulin deficiency; also if insulin is low then uptake of glucose to tissues is poor so fats used as fuel instead Eg Sulphuric acid (from the metabolism of proteins; ie Cys has sulphur atoms) Compensation: May be “partial” or “complete” compensation Compensation is always by the opposing system (resp/metabolic) to the one giving the initial deviation Respiratory compensation is quick whereas renal compensation takes longer (therefore can distinguish acute and chronic respiratory acid/alkylosis) 69 Giles Kisby GE Y1 Respiratory o Bicarbonate reading; Is derived from the 3x electrode readings using the Henderson Hasselbach equation: 0.23 is the solubility coefficient of CO2 (when the pCO2 is in kPa) [ie the coefficient / constant for pCO2 conversion to H2CO3] pKa of HCO3- is 6.1 Normal range is 22-26 mEq o - Base excess: Gives an indication as to the proportional contribution of metabolic factors to the overall acid/base disturbance Theoretical HCO3- level based on paCO2 is calculated (assuming no renal or metabolic disturbance); Then the difference between this value and the actual HCO3- value is determined a positive value means a base (ie HCO3-) excess (see below for causes) Base excess normal range = -2 +2 mmol/L Base excess beyond the reference range indicates o metabolic alkalosis if too high (more than +2 mEq/L) o metabolic acidosis if too low (less than −2 mEq/L) NOT ALWAYS: IS TRUE THAT MEANS THAT THE HCO3 FORMED FROM THE LEVEL OF CO2 IS BEING GREATLY LOST BUT THIS MAY BE PHYSIOLOGICAL EG INSTEAD COULD JUST BE RENAL COMPENSATION FOR A RESP ALKYLOSIS AS BELOW: 70 Giles Kisby - - GE Y1 Respiratory A high base excess, thus metabolic alkalosis, usually involves an excess of bicarbonate. It can be caused by o Compensation for primary respiratory acidosis o Excessive loss of HCl in gastric juice by vomiting [so more H+ secretion to stomach which is coupled to HCO3- excretion to blood] o Renal overproduction of bicarbonate, in either contraction alkalosis or Cushing's/Conn’s disease A base deficit (a below-normal base excess), thus metabolic acidosis, usually involves either excretion of bicarbonate or neutralization of bicarbonate by excess organic acids. Common causes include o Compensation for primary respiratory alkalosis o Diabetic ketoacidosis, in which high levels of acidic ketone bodies are produced o Lactic acidosis, due to anaerobic metabolism during heavy exercise or hypoxia o Chronic renal failure, preventing excretion of acid and resorption and production of bicarbonate o Diarrhea, in which large amounts of bicarbonate are excreted o Ingestion of poisons such as methanol, ethylene glycol, or excessive aspirin o Metabolic alkalosis: Subject may be vomiting up stomach contents 71 Giles Kisby GE Y1 Respiratory o Antacids given for indigestion may cause this state Hyperventilation: [ ~ acute respiratory alkalosis]: [get subject to breath into paper bag to break the cycle] o Acute respiratory acidosis: Patients with COPD etc build up resistance to having a high paCO2 so that it no longer acts as the driver for increased ventilation – instead low paO2 becomes the driver (= hypoxic drive); Therefore if patient is given oxygen their RR will fall considerably giving acute respiratory acidosis Heroin is another cause of low RR which then would lead to respiratory acidosis [naloxone is an opioid antagonist drug used to counter heroin effects] Examples: 72 Giles Kisby GE Y1 Respiratory [ie low RR is due to having COPD etc but being put on oxygen: see explanation above] 73 Giles Kisby GE Y1 Respiratory - Pulse oximetry: o Measures SaO2 from surface o Measured by absorption spectra (spec of Hb and deoxyHb differ); reading comparison with pulse variations in absorbance allow exclusion of absorbances by other tissues that would otherwise contribute to the reading o If <95% then need a fitness to fly test (cabin pressure equates to being at 8000ft so less oxygen (lower partial pressure because lower OVR pressure) o <90%: home O2 prescribed o <87%: emergency - Types of resp failure: [covered properly later; this is from wiki] o Type 1: Type 1 respiratory failure is defined as hypoxia without hypercapnia, and indeed the PaCO2 may be normal or low. It is typically caused by a ventilation/perfusion (V/Q) mismatch; the volume of air flowing in and out of the lungs is not matched with the flow of blood to the lungs. Eg alveoli filling with debris etc but CO2 is relatively easy to remove from the blood so normal levels (or low levels if patient is reacting to low O2 with high RR) Eg. Parenchymal disease (V/Q mismatch) Diseases of vasculature and shunts: o right-to-left shunt [presumably unlikely to be severe enough to rise CO2 significantly], o pulmonary embolism Interstitial lung diseases: ARDS, [pneumonia]. o Type 2: Hypoxia with Hypercapnia Type 2 respiratory failure is caused by inadequate ventilation; both oxygen and carbon dioxide are affected. Defined as the build-up of carbon dioxide levels (PaCO2) that has been generated by the body. The underlying causes include: Increased airways resistance (chronic obstructive pulmonary disease, asthma, suffocation) Reduced breathing effort (drug effects, brain stem lesion, extreme obesity) A decrease in the area of the lung available for gas exchange (such as in chronic bronchitis). Neuromuscular problems (GB syndrome.,[1] myasthenia gravis, motor neurone disease) Deformed (kyphoscoliosis), rigid (ankylosing spondylitis), or flail chest MISC UNRELATED UNEXAMINABLE: [NAVY mnemonic]: 74 Giles Kisby GE Y1 Respiratory Misc from internet: • TLCO = transfer factor for the lung for carbon monoxide i.e. Total diffusing capacity for the lung – Same as DLCO • KCO = transfer coefficent i.e. Diffusing capacity of the lung per unit volume, standardised for alveolar volume (VA) • VA = Lung volume in which carbon monoxide diffuses into during a single breath-hold technique • Low TLC: Low TLCO and low/normal KCO = intrapulmonary restrictive defect • – Interstitial lung diseases e.g. Idiopathic pulmonary fibrosis, sarcoidosis, CTD, HP – Cardiac e.g. Pulmonary oedema – Pulmonary vascular disease e.g. Pulmonary hypertension (may have normal TLCO) High TLC: Low TLCO + KCO 75 Giles Kisby GE Y1 Respiratory – emphysema (in the context of obstruction) 76 Giles Kisby GE Y1 Respiratory 26/11/13: Respiratory physiology and diving: Dr Peter Wilmshurst Los from booklet: -respiratory physiological principles are reinforced and modified by hyperbaric conditions Notes: - Hydrostatic effects of diving: Immersion in warm water: o Increases venous return and central blood volume by 500ml and reduces lung volumes (due to the weight of the water compressing tissues and blood) Increases Right atrium and Pulmonary artery pressures by 15-20mm Hg Increases stroke volume and cardiac output by 20% (because of the frankstarling law) The cold (if the water is cold) will supplement these effects via sympathetic nervous signalling Can lead to pulmonary edema (the commonest cause of death in divers but can be misinterpreted as having drowned at post mortem) o Causes natriuresis [process of excretion of sodium in the urine; lowers the concentration of sodium in the blood and also tends to lower blood volume because osmotic forces make water follow sodium out of the body's blood circulation and into the urine] and diuresis [increased urine production] o [has been used in the past to help ease symptoms of peripheral edema] o Sudden lifting out water vertically after long period will give pooling of blood in legs (as body has been trying to vasodilate); this loss of blood from brain can cause death - Effects to lung volume and partial pressure of gases (nitrogen and oxygen) in the lungs: 77 Giles Kisby GE Y1 Respiratory o o Lung volume is divided by the number of “Bar” exerted: Ie VC, FRC, etc reduced Will become negatively buoyant as lungs are less full (minor supplementary contributor: the gases in them are at a raised pressure) Compression of gas spaces can cause barotrauma (lung squeeze, middle ear, sinuses) Expansion of gas can cause barotrauma on ascent Partial pressure of a given gas at a given initial value is multiplied by the number of bar exerted: Means that breathing takes more work as are moving gases of higher pressures [Denser gas increases work of breathing for ambient pressure divers; nb Boyle’s law: pressure exerted by a given mass of gas is inversely proportional to its volume] This is one reason for mixing the O2 with helium instead of N2 (to maintain lower pressure of the gas) The higher partial pressure of N2 in the lungs means that far more N2 becomes dissolved in the blood (ie the P(N2) is the key determinant of amount that will be transferred to blood) N2 is fat soluble and not inert at high partial pressures: o 30m on air – mild euphoria o 40-50m on air – impaired responses, mathematical errors o 50m on air - max for commercial air dives o 50-70m on air – confusion, drowsiness o 70-90m on air – hallucinations, loss of consciousness 78 Giles Kisby GE Y1 Respiratory - This N2 in the blood can then be liberated from solution at tissues/blood upon ascent as bubbles [like releasing pressure when opening a fizzy drink]; gas bubbles are usually tolerated well by the body and absorbed readily at tissues and at the lungs but tissues are likely supersaturated with N2 upon diving so bubbles can persist; the bubbles can give gas embolism [or in eye can give bubbles between contact lens and cornea giving temporary cornea damage] In contrast, the amount of O2 in blood does NOT change significantly due to depth because its presence in blood is determined by carrier molecules (Hb) and these are usually already fully saturated; does however mean that the tanks can be filled with a far lower concentration of O2 than would be required for breathing at the surface NB O2 at partial pressure of >1.6 bar can have toxic effects [presumably due to some increase in dissolved O2 in blood]: o air at 70m: can cause convulsions “Decompression illness”: [ie disease from decompression due to the above phenomena] o Causes of decompression illness Unsafe dive profile – rapid ascent or missed decompression stops [pneumothorax, etc could result] Shunt-mediated with paradoxical gas embolism – venous bubbles pass to the systemic circulation – slightly delayed onset Ie PFO (patent foramen ovale) lets the bubbles into the systemic arterial circulation (if had just gone to lungs would have just been absorbed out of the blood there); ~25% of people have PFO but for arterial bubbles will need a right to left shunt too – present in ~2.5% of people (then gas embolism); the bubbles are formed on the venous side as during ascent the arterial blood has just come from lungs so contains a suitable amount of N2 for that ambient pressure – the venous blood has come from the tissues and so reflects suitable N2 content for some time ago (this delay means that upon ascent veins have too much N2 for that depth; therefore decompression stops are essential) Lung disease causing pulmonary barotrauma – very rapid onset [eg a slight pneumothorax / any gas trapping could lead to barotrauma upon ascent] o Types of decompression illness Neurological – most frequent in amateur divers Cardiovascular – uncommon but most frequently fatal Joint pains – most common in caisson workers [underwater tunnel building: have to work in pressure chambers to avoid water inflow to work area] 79 Giles Kisby GE Y1 Respiratory - Cutaneous Breath-hold diving o Hypercapnia Is the normal reason for breaking the breath-hold (CO2 level regulates breathing) o Hyperventilation Does nothing/little to help O2 saturation (already maximal) but pushes down CO2 levels so takes longer to get the urge to breathe However, upon ascent after hyperventilation when O2 needed most and is already low in lungs, the P(O2) in lungs will plummet as ambient pressure drops blood O2 levels drop further unconsiousness o Compression of gas spaces in middle ear giving pain limits record attempts but 214m free-dive is the record o Negative buoyancy experienced due to loss in lung volume / in in gas pressure upon descent (so difficult to swim back up) 80 Giles Kisby GE Y1 Respiratory 27/11/13: Helium Dilution and Other Lung Function Measurements: Hannah Tighe Los from booklet: be measured by simple spirometry can’t be measure by simple spirometry trapping in COPD) Notes: - Volumes and capacities: - Lung Function: o Primary function of Lung is gas exchange o Interaction of 3 processes: Ventilation Diffusion 81 Giles Kisby GE Y1 Respiratory o - Perfusion Lung Function Tests used to aid diagnosis; assist with prognosis and monitor progression of disease and response to interventions Lung Disease o Obstructive o Restrictive o Mixed o Differentiate between these disease types using the following investigations: - Spirometry: o Useful tool for monitoring disease progression in chronic conditions o VC gives us a useful indication of the ability of the lung to expand o For judging overall lung volume is not good enough on its own (there may be a big/small RV?; reading may be inaccurate for many patients due to onset of coughing towards end of breath (esp for airflow obstruction patients giving underestimates) [for these reasons must measure TLC and RV (can’t be obtained by spirometry) for important additional info as to what is occurring in the lungs] - Variations in compliance: [nb elastin is involved in expansion and recoil] o Compliance varies through inhaled volume: decreases towards end of inspiration so takes more energy per unit volume inhaled as an increasing change in pressure is required for each additional unit volume In a normal lung breathing typically occurs in the lower 70% of the curve (ie where breathing takes relatively little energy) [Little change in pressure is needed to expand lung at normal tidal levels] o Compliance varies with diseases: High in emphysema [elastic properties of alveoli are lost and lungs become large and floppy], low in fibrosis [lungs stiffen and are harder to inflate]: 82 Giles Kisby GE Y1 Respiratory - How do we measure TLC and RV? o Gas dilution o Plethysmography o Gas washout - Gas dilution o Principle: 1. Gas mixture with known concentration of a tracer gas breathed in over time [eg 3.5L of 10% He] NB Patient is connected to the system at FRC, not RV. This is so that the patient can breathe comfortably at that volume (important for mixing); ie value found will be FRC which then has ERV subtracted (from simple spirometry results) to give the RV 2. Helium (He) is used: Not found in room air Inert Will not diffuse out of the lung 3. Mixes with air in lungs [patient breathes freely to allow mixing] 4. Once equilibrated, expired gas concentration measured [Final [He] measured] 5. Volume of gas in lungs calculated from dilution effect Calculated using mass balance [OVR mass of He remains constant; Mass = Concentration x Volume] Eg. Mass of He before mixing: [mass in lung] + [mass in spirometer]: (0*RV) + (10*3.5) [ie conc * vol = mass (if conc in mass per vol)] Mass of He after mixing: [mass in lung] + [mass in spirometer]: (5*RV) + (5*3.5) or 5*(RV+3.5) No He is lost, so RV = 3.5L [nb as above the calculations in reality will be working out FRC which then has ERV subtracted (from simple spirometry) to give the RV] 83 Giles Kisby GE Y1 Respiratory - Interpreting lung volumes (TLC and RV) o Low TLC and RV Restriction [ie due to reduces IRV] / small lungs o Normal TLC and RV normal lungs o Raised TLC and RV Can be normal; hyperinflation and gas trapping [ie in obstructive disease despite reduced FVC & ERV, an RV rise matches the ERV fall and the increased use of “top of lungs” can lead to hyperinflation (ie elevated TLC) - Whole Body Plethysmography (Body box) o Based on Boyle’s Law, uses pressure and volume relationship to calculate TGV (= FRC) o Boyle's Law is used to calculate the unknown volume within the lungs. First, the change in volume of the chest is computed. The initial pressure and volume of the box are set equal to the known pressure after expansion times the unknown new volume. Once the new volume is found, the original volume minus the new volume is the change in volume in the box and also the change in volume in the chest. With this information, Boyle's Law is used again to determine the original volume of gas in the chest: the initial volume (unknown) times the initial pressure (as recorded for the box) is equal to the final volume (calculated as above) times the final pressure (as recorded for the box). o Not suitable if the patient v obese, bed bound, claustrophobic - Nitrogen Washout o At FRC (unknown vol), air in lungs is ~80% Nitrogen; If we can measure volume of N2 then can calculate FRC o A subject takes a breath of 100% oxygen and exhales through a one-way valve measuring nitrogen content and volume. A plot of the nitrogen concentration (as a % of total gas) vs. expired volume is obtained by increasing the nitrogen concentration from zero to the percentage of nitrogen in the alveoli. The nitrogen concentration is initially zero because the subject is exhaling the dead space oxygen they just breathed in (does not participate in alveolar exchange), and climbs as alveolar air mixes with the dead space air. o Most people with a normal distribution of airways resistances will reduce their expired end-tidal nitrogen concentrations to less than 2.5% within seven minutes. Individuals with low lung volume can take longer than seven minutes to remove all the nitrogen 84 Giles Kisby GE Y1 Respiratory - Lung volumes measurements will differentiate between normal and restrictive lung disease [ie gives us info on ventilation but not on diffusion and perfusion; see below sections for this] o If patient has normal lung volumes but has other resp symptoms eg dyspnoea or reduced exercise tolerance (SOBOE) then more information is needed o If patient has reduced lung volumes, then we need to know why - Transfer Factor (TL) [sometimes called “diffusion capacity”] o Aim: To measure rate of oxygen transfer from lungs to capillary blood; TL is the ability of the lung to transfer gas [considers diffusion and perfusion] o Depends on amount of functioning capillary bed and its contact with ventilated alveoli [Fick’s Law of Diffusion: surface area and thickness and pressure gradient] o Reflects presence of pulmonary vascular and parenchymal disorders o TL = K x VA 1. Rate of diffusion (with respect to time) per litre alveolar volume (K) Ie a measure of each individual section of lung (per unit alveolar volume) to transfer gas To estimate K: use a marker gas that diffuses out of lungs and behaves ‘like’ oxygen – carbon monoxide (CO) o Rate of reaction of CO with Hb similar to O2 o O2 and CO bind to same site on Hb molecule o In normal non-smokers endogenous CO levels are negligible o Low [CO] are easily analysed using infra-red gas analysis o Use of CO in trace quantities does not harm subject 2. Alveolar volume (VA) Ie the volume available; is just referring to the volume / SA available – does not necessarily mean that good OVR diffusion is occuring To estimate alveolar volume (VA): requires an insoluble tracer gas; often He or Methane o Technique to measure TL: o Patient exhales to RV o Inhale (to TLC) gas mixture containing 0.3% CO (+ 0.3% CH4) o Holds breath for up to 10 seconds to allow transfer of gas from Lung to Capillaries o Breathes out (not necessarily to RV) o Difference in conc CO of the before and after breath-hold gives us a measure of the TL o Initial portion of exhaled air is ‘washout’ and not use for analysis [was not used in gas exchange] o Non-lung-disease factors affecting diffusion: Hb concentration anaemia/menstruation/polycythaemia [high concentration of red blood cells in your blood] for accurate results should correct for Hb 85 Giles Kisby GE Y1 Respiratory o - Exercise increased SA for gas exchange (inc ventilation) and in capillary blood volume (inc CO) for accurate results should do at rest Smoking Raised endogenous CO for accurate results should ask patient not to smoke beforehand / correct for the CO Interpreting Transfer Factor Emphysema and Fibrosis both have reduced TL but for different reasons….. Remember: TL = K x VA If TL is reduced, you must look at K and VA to determine why: only Kco reduced for emphysema, both Kco and VA reduced for fibrosis (see below for detail) Emphysema patient: o Spirometry FEV1: FVC: VC: o 0.83 22% = very very low 2.90 63% (ie fast VC) = very low 4.10 86% (ie slow VC) = low Can tell at this point that there is likely some form of obstructive, small airways disease as VC – FVC > 500ml (would be more < 500ml for normal / restrictive) and low FEV1 (would be normal/high for normal / restrictive) o However cannot be certain it’s an obstructive disease at this stage due to potential for inaccuracies in spirometry due to patient behaviour – eg may just have tendancy to start coughing when breathing out fast PV loop: 86 Giles Kisby GE Y1 Respiratory o o Loop (on right) shows that upon rapid exhale the airways shut very quickly giving deviation from the normal graph shape (become much flatter compared to norm) shown in grey – this indicates small airways disease (aka obstructive). o If did this second now have enough information to confidently diagnose obstructive airway disease / COPD (of which emphysema is just one option) Loop is also of decreased area indicating reduced FVC (could potentially be indicating Obstructive or restrictive) Lung Volumes TLC: 8.08 118% RV: 3.98 199% TLC is mildly raised (can occur when are working at top of lungs for long period such as in an obstructive disease) RV is very high reflecting the fact that the patient can’t get the air out very well due to obstructive disease; o If did this second, now have enough information to confidently diagnose obstructive airway disease / COPD (of which emphysema is just one option) o SOBOE experienced will be due to: Narrowing of the airways Having to breath at top of lungs (which requires greater work as discussed prev) RV / TLC > 30/40% is a further indicator of obstructive airways disease Diffusion TLco: 5.07 48% Kco: 0.80 52% VA: 6.33 103% 87 Giles Kisby GE Y1 Respiratory - TLco is low but must look to see what the reason for this is: here is due to normal VA but low Kco allowing us to make a more specific diagnosis than just obstructive – is Emphysema because this gives retained VA (retained volume and surface area of the lungs) but loss of diffusion capacity due to loss of alveolar-capillary membrane surface area [ie is a loss of function within the tissue] VA should be ~90% of TLC but in this case is lower indicating that there is a lot of lung not being used (ie indicated the same thing as the direct high RV measurement) Pulmonary Fibrosis patient: [in this case the fibrosis is secondary to bleomycin chemotherapy] o Spirometry FEV1 0.97 37% FVC 1.11 36% VC 1.20 39% All are reduced but have reduced in line with each other so FEV1/FVC will be normal meaning is unlikely to be obstructive but strong indication that it is a restrictive condition o PV Loop: o o PV loop is of the correct shape but is smaller than for a normal patient so is a normal exhale for a lung of that size but the lung is small (due to the restrictive disease giving a low IRV) Lung Volumes TLC 2.17 46% RV 0.84 55% Both TLC and RV are reduced indicating a small lung (due to the restrictive disease giving a low IRV) [note that Rv has fallen to generate space more space at the bottom of the lungs for breathing Diffusion TLco 1.35 16% 88 Giles Kisby GE Y1 Respiratory Kco Va - Lobectomy patient: o Spirometry FEV1 FVC VC o 1.46 43% 1.88 48% 1.87 47% All are reduced but have reduced in line with each other so FEV1/FVC will be normal meaning is unlikely to be obstructive but strong indication that it is a restrictive condition; HOWEVER, in this case isn’t a restrictive disease – due to a lobectomy instead [not a restrictive lung disease - does not increase the work of breathing] PV loop: o 0.64 37% 2.11 50% TLco is low because BOTH Kco AND VA are low o VA is low due to the restrictive disease giving a low IRV; VA is >90% of TLC so we know that the loss of VA is due to loss of volume at the top of the lung not the bottom o Kco is low and we are told in this case that the restrictive lung disease is pulmonary fibrosis so we can deduce that the reason for the low Kco is thickening of the alveolar membrane where gas exchange occurs Lung Volumes TLC RV PV loop is of the correct shape but is smaller than for a normal patient so is a normal exhale for a lung of that size but the lung is small (due to the restrictive disease giving a low IRV); HOWEVER, in this case isn’t a restrictive disease – small lung is due to a lobectomy instead 3.00 0.90 52% 52% 89 Giles Kisby GE Y1 Respiratory o Diffusion TLco Kco VA Both TLC and RV are reduced indicating a small lung (due to the restrictive disease giving a low IRV) [note that Rv has fallen to generate space more space at the bottom of the lungs for breathing; HOWEVER, in this case isn’t a restrictive disease – small lung is due to a lobectomy instead 4.05 41% 1.55 91% 2.62 51% TLco is low because VA is low: o VA is low due to the restrictive disease (however in this case is lobectomy) giving a low IRV; VA is >90% of TLC so we know that the loss of VA is due to loss of volume at the top of the lung not the bottom Ie mimics the results of mechanical restrictive lung disease (see below) - There are two subsets of restrictive lung disease: Mechanical: o Eg muscle disruption, Eg. Kyphoscoliosis o Kco NOT reduced Parenchymal: o Eg. Pulmonary fibrosis o Kco reduced - Wegener’s Granulomatosis patients (form of vasculitis; inflam of blood vessels; cough up blood etc): [normally do not analyse patients coughing up blood (haemoptysis) because may be TB so infection risk] o Spirometry FEV1 1.91 85% FVC 2.45 81% VC 2.52 81% This patient is a smoker so spirometry readings are a little low o Diffusion TLco 8.81 127% Kco 2.31 198% VA 3.82 71% TLco high due to high Kco; because blood has escaped blood vessels in the lungs it is in better contact with the air so more efficient transfer [this is despite a drop of Hb through time in the blood as blood is lost from the body] 90 Giles Kisby GE Y1 Respiratory The blood may be contributing to the slightly lower VA reading than normal by taking up space Treatment: Treat the inflammation and the lung function / integrity return to normal in most cases If very severe can embolise the bleeding (prob means use a coil) 28/11/13: The Pulmonary circulation: Dr Luke Howard Los from booklet: Lecture 6: Pulmonary Circulation I. (Dr Claire Shovlin, c.shovlin@imperial.ac.uk) At the end of this lecture you should be able to: • Compare the systemic and pulmonary circulations with respect to • (i) the structure of the arteries and arterioles • (ii) the mean arterial blood pressure and • (iii) the overall resistance to blood flow. • Explain how differences in the arterial blood pressures of the two circulations influence the structure of the two ventricles of the heart. • Describe and explain the relative difference in blood flow to the bases and apices of the lungs in a standing human. • Explain, with reference to the pulmonary circulation, the meaning of the terms vascular recruitment and hypoxic vasoconstriction. • Explain the importance of hypoxic vasoconstriction in the fetus. Give one advantage and one disadvantage of this response in an adult suffering from chronic lung disease • Understand the route of fluid flux from pulmonary capillaries to lymphatics Los from slides: • • • • Compare the systemic and pulmonary circulations with respect to (i) the structure of the arteries and arterioles (ii) the mean arterial blood pressure and (iii) the overall resistance to blood flow. Explain how differences in the arterial blood pressures of the two circulations influence the structure of the two ventricles of the heart. Describe and explain the relative difference in blood flow to the bases and apices of the lungs in a standing human. Explain, with reference to the pulmonary circulation, the meaning of the terms vascular recruitment and hypoxic vasoconstriction. 91 Giles Kisby • • • • • GE Y1 Respiratory Explain the importance of hypoxic vasoconstriction in the fetus. Give one advantage and one disadvantage of this response in an adult suffering from chronic lung disease Give two reasons why lung disease may lead to pulmonary hypertension. Explain what is meant by “cor pulmonale”. Explain what is meant by pulmonary oedema. Identify 3 pathophysiological mechanisms that may lead to this state. Explain the term “pulmonary embolism” and state the typical site of origin of such emboli. Describe the consequences of a large embolus with respect to i) the right side of the heart and the pulmonary circulation, ii) the viability of the lung tissue and iii) the implications for gas exchange. Appreciate, in the context of the pulmonary circulation, the concept of shunting. Identify the potential deleterious effects of an increased pulmonary shunt. Notes: - General: o 1% of systemic cardiac output is used to supply the lungs (high) o Arterial supply is from the bronchial arteries derived from the aorta o Drainage is via bronchial veins Some anastomoses exist between bronchial and pulmonary blood vessels but mainly on the venous side (not arterial) - Functions of the pulmonary circulation: o 1. Gas exchange o 2. Filtering of small emboli (blood clots, air, fats) from the circulation o 3. Metabolism of vasoactive substances ACE present to perform AIAII Bradykinin inactivation 100% removal of serotonin from circulation 30% removal of adrenaline from circulation Removal of prostaglandins and leukotrienes - Embryology: o Ductus arteriosus [anatomical shunt] In the full term infant, closure of the ductus arteriosus occurs in two phases: (1) ‘functional’ closure of the lumen within the first hours after birth by smooth muscle constriction (Patency of the fetal ductus arteriosus is regulated by both dilating and contracting factors. The ductus normally has a high level of intrinsic tone during fetal life. The factors that promote ductus constriction in the fetus have yet to be identified) and (2) anatomic occlusion over the next several days due to extensive thickening and loss of smooth muscle cells from the inner muscle media 92 Giles Kisby GE Y1 Respiratory o o o Nb also, the hypoxic pulmonary vasoconstriction occurring in the foetus will end when the baby takes its first breath – this prob plays a role in triggering the above responses Foramen ovale [anatomical shunt] Closure will occur when the heart beat replaces placenta supply because now the left heart pressure is higher than on the right holding the valve shut Less than 10% of blood from heart will go through the lungs in foetus with rest passing systemically via the shunts Per cycle ~60% of blood is channelled through the placenta for oxygenation with the rest passing systemically back to the heart - Shunts in adult: o Physiologic shunt About 2% of the cardiac output normally bypasses the alveoli Thesbian veins, bronchial blood Could be considered V/Q defects though are normal o right to left shunts: [anatomical]: don’t oxygenate a proportion of the blood so saturation level can drop – not a problem unless v severe: ie hypoxemia occurs O2 provision won’t help but may be useful in indicating severity / diagnosing however the main problem is the reduced filtering of the blood: greater risk of stroke etc give V/Q mismatching / defect o Left-to-right shunts do not cause hypoxemia do not cause V/Q mismatching/defect Among the causes of left-to-right shunts are patent ductus arteriosus and traumatic injury - lung circ characteristics: o The lungs have to accommodate the same C.O. as the rest of the body put together due to it being a double circulation The difference is a result of a small amount of coronary venous blood that drains directly into the left ventricle through the thebesian vein The reason that pulmonary blood flow can be equal to systemic blood flow is that pulmonary pressures and resistances are proportionately lower than systemic pressures and resistances o High capacity in pulmonary circulation: The lungs normally have spare capacity: Eg Exercise or Fever (also known as pyrexia or febrile response) will give increases to CO that the lungs must be able to accommodate. Similarly, blocks of lung areas due to filtration roles will demand other vessels to take up the slack o Low resistance in pulmonary circulation: has to be relatively passive to be compatible with supply from systemic: 93 Giles Kisby GE Y1 Respiratory o Eg Exercise or Fever (also known as pyrexia or febrile response) will give increases to CO that the lungs must be able to accommodate. Similarly, blocks of lung areas due to filtration roles will demand other vessels to take up the slack However, active mechanisms do exist to give certain regulation of bloodflow in the lungs passive by gravity and recruitment/distension (cf) active by hypoxic pulmonary vasoconstriction (HPV) (cf) PVR = pulmonary vascular resistance = (15-5)/5 = 2 mmHgL-1min Compared to SVR (= systemic vascular resistance) = 18 mmHgL-1min [ie CO=5Lmin-1 as a standard value] Pulmonary systolic = RV systolic as are connected during this time but the diastolics are not equal as the valves will be closed mmHgL-1min = wood units The lungs are a low pressure system: Peak ~34cmH2O (25mmHg) of pressure in lungs compared to ~163mmH2O in systemic (120mmHg) Pulmonary Systemic Nb diastole longer than systole so mean is not average of values Nb in below diagram the mean systemic should be 93mmHg insteadcirculation circulation 25 (mean 15) 8 120 (mean 100) 80 artery artery RV 25/0 RA 2 vein LV 120/0 LA 5 vein Low pressure together with gravity considerations means that the bottom of the lungs is more perfused than higher up (except for the very bottom which is squashed between diaphragm and weight of lung) 94 Giles Kisby GE Y1 Respiratory o o - pulmonary arteries/ veins have thinner walls and less smooth muscle compared to systemic Unlike the systemic capillaries, which are often arranged as a network of tubular vessels with some interconnections, the pulmonary capillaries mesh together in the alveolar wall so that blood flows as a thin sheet / mesh Differences in blood flow: o The distribution of pulmonary blood flow within the lung is uneven and the distribution can be explained by the effects of gravity. o When a person is supine, blood flow is nearly uniform, since the entire lung is at the same gravitational level. o However, when a person is upright, gravitational effects are not uniform, and blood flow is lowest at the apex of the lungClose proximity of alveoli and capillaries for gas exchange results in exposure of vessels to alveolar pressure (pA) o Zone 1: pA > pa > pv high in lungs so no blood flow This compression will cause the capillaries to close, reducing regional blood flow. Normally, in zone 1, arterial pressure is just high enough to prevent this closure, and zone 1 is perfused, albeit at a low flow rate. However, if arterial pressure is decreased (e.g., due to hemorrhage) or if alveolar pressure is increased (e.g., by positive pressure breathing - CPAP), then PA will be greater than Pa, and the blood vessels will be compressed and will close. Under these conditions, zone 1 will be ventilated but not perfused. There can be no gas exchange if there is no perfusion, and zone 1 will become part of the physiologic dead space. o Zone 2: pa > pA > pv mid way down lungs so moderate & rapidly increasing blood flow through this region Although compression of the capillaries does not present a problem in zone 2, blood flow is driven by the difference between arterial and alveolar 95 Giles Kisby GE Y1 Respiratory o - pressure, not by the difference between arterial and venous pressure (as it is in systemic vascular beds). Zone 3:pa > pv > pA low in lungs so high & slowly increasing blood flow through this region (until very base of lung where relationship lost due to compression) Blood flow in zone 3 is driven by the difference between arterial pressure and venous pressure, as it is in other vascular beds Accommodation of increased cardiac output: o Two main methods of maintaining a low PAP (pulmonary arterial pressure) or PVR (pulmonary vascular resistance) upon increasing CO burden (eg pneumonectomy, exercise) Ie CO inc (or same CO to reduced lung region in the case of pneumonectomy) BP inc recruitment / distention Recruitment of unused capillary vessels (inc in pa gives transition of upper lung regions from zone 1 to zone 2 as above) 96 Giles Kisby GE Y1 Respiratory - Distension of capillary vessels already in use (inc in CO gives inc in pa and pv so blood vessels transition in zone 3 direction) Ventilation – perfusion matching (and mismatching): o At rest VA (alveolar ventilation) and Q (Blood flow (~perfusion)) are the same: 5Lmin-1 [actually the ratio is 0.8] Written: The value of 0.8 for V_/Q_ is an average for the entire lung. In fact, in the three zones of the lung, V/Q is uneven, just as blood flow is uneven. As already described, regional variations in pulmonary blood flow are caused by gravitational effects: Zone 1 has the lowest blood flow, and zone 3 the highest. Alveolar ventilation also varies in the same direction among the zones of the lung [accordion analogy: the weight of the accordion (lung) squeezes air out of the bellows at the base, and most of the FRC fills the bellows at the apex. When the next breath is taken, most of the potential space to be ventilated is at the base of the lung, while the apex is already full Ventilation is lower in zone 1 and higher in zone 3] However, regional variations in ventilation are not as great as regional variations in blood flow. Therefore, the V/Q ratio is highest in zone 1 and lowest in zone 3 In addition to this source of uneven matching there the anatomical shunts may also be thought of as relevant to V/Q mismatching as they (thesbian veins, RL shunts and bronchial blood flow) are examples of perfusion not being given/matched to a ventilation o [these are VQ shunts but prob not considered in the 0.8 figure as this is for total lung perfusion and total ventilation figures – this fiqure is for healthy lung anyway so definitely wouldn’t be considering RL shunts] Regulation of Pulmonary Blood Flow In the lungs hypoxic vasoconstriction occurs as an adaptive mechanism, reducing pulmonary blood flow to poorly ventilated areas where the blood flow would be “wasted.” o Useful in localised diseases; The compensatory mechanism fails, however, if the lung disease is widespread o Altitude/breathing low O2 mixture: The low PAO2 produces global vasoconstriction of pulmonary arterioles and an increase in pulmonary vascular resistance. In response to the increase in resistance, pulmonary arterial pressure 97 Giles Kisby GE Y1 Respiratory - increases. In chronic hypoxia, the increased pulmonary arterial pressure causes hypertrophy of the right ventricle, which must pump against an increased afterload. o Occurs in foetus Thromboxane A2, a product of arachidonic acid metabolism (via the cyclooxygenase pathway) in macrophages, leukocytes, and endothelial cells, is produced in response to certain types of lung injury. Thromboxane A2 is a powerful local vasoconstrictor of both arterioles and veins. Prostacyclin (prostaglandin I2), also a product of arachidonic acid metabolism via the cyclooxygenase pathway, is a potent local vasodilator. It is produced by lung endothelial cells. V/Q mismatch occurs in one of two situations: 1. A section of lung is ventilated but not perfused. 2. A section of lung is perfused but not ventilated. Extreme alterations of V/Q An area with no ventilation (and thus a V/Q of zero) is termed "shunt." [nb giving a patient oxygen will not help with this] o Shunt is illustrated by airway obstruction and right-to-left cardiac shunts. An area with no perfusion (and thus a V/Q undefined though approaching infinity) is termed dead space. o Dead space is illustrated by pulmonary embolism Global high VQ if heart failure Focal high VQ if embolism shunt and dead space can coexist: In some lung diseases, the entire range of possible V/Q defects is exhibited Eg pneumonia / edema gives fluid in alveoli: Pneumonia / edema gives a shunt (here is used to refer to a physiological shunt rather than an anatomical one; refers to the blood passing through the alveoli but not being oxygenated because of fluid in alveoli) Result is a fall in ventilation but not perfusion giving mismatch and low VA/Q A pulmonary shunt is a physiological condition which results when the alveoli of the lungs are perfused with blood as normal, but ventilation (the 98 Giles Kisby GE Y1 Respiratory - supply of air) fails to supply the perfused region. In other words, the ventilation/perfusion ratio (the ratio of air reaching the alveoli to blood perfusing them) is zero. A pulmonary shunt often occurs when the alveoli fill with fluid, causing parts of the lung to be unventilated although they are still perfused. Intrapulmonary shunting is the main cause of hypoxemia (inadequate blood oxygen) in pulmonary edema and conditions such as pneumonia in which the lungs become consolidated Hypoxic pulmonary vasoconstriction occurs (HPV) 1. Alveolar oxygen tension falls 2. Response of lungs is active (ie lung role is not just passive) vasoconstriction of pulmonary arteries that are <1000µm in diameter (ie the small arteries) occurs o Immediate response is achieved by the inhibition of K+ channels that occurs under hypoxic conditions; this depolarisation gives calcium influx and therefore smooth muscle contraction [note that pulmonary artery myocytes are simiar to those of carotid body and not like those of other SM cells (at all other locations hypoxia inhibits contraction of SM cells)] o Longer term response is achieved involving HIFs (hypoxyinducible factors) which under hypoxic conditions alter gene expression such as to restore adequate oxygenation of the body (not just active in the lungs) [this HIF action can lead to permanent remodelling of tissues] 3. Bloodflow is diverted to other lung regions experiencing a greater O2 conc o Nb both PAP and hypoxia/aeration influence bloodflow: both worsen bloodflow but PAP OVR beneficial as decreases shunting by recruiting squashed alveoli at bottom of lung Note that HPV is problematic when occurs to whole lung such as due to hypoxia at high altitude [may be useful to look at the cardio notes on high altitude which were taken off the cardio learning objectives] Pulmonary edema: o Interstitial space is normally a potential space but becomes filled with fluid when the balance of fluid exit from the blood, fluid return to the blood and lymphatic drainage is lost: o “Starling forces”: 99 Giles Kisby GE Y1 Respiratory o o o o - The first phase of pulmonary edema is interstitial edema The second phase of pulmonary edema is alveolar flooding Note that the harder work breathing will give greater negative pressures exerted and so worsen the edema by increasing fluid exit Effects: Impaired gas exchange Reduced lung compliance (wet lungs are stiff lungs) Increased pulmonary artery pressure (prob via hypoxic vasoconstriction) Clinically: Terrified patient Severe breathlessness Pink frothy sputum Crackles on auscultation Causes: High hydrostatic pressure: [~high pulmonary venous pressure] Left heart failure Mitral stenosis Low plasma colloid pressure [~low plasma pr ot conc] Starvation Abnormal leakage out at kidney or gut High capillary permeability [~endothelial cell damage] ARDS [adult respiratory distress syndrome Pulmonary embolus: o Can scan for ventilated and perfused areas of the lung separately to allow identification of VQ mismatches o Fat / air / blood clot o Spectrum exists: Normal filtration: spare capacity allows toleration of small emboli unless is chronic and the spare capacity is overwealmed Moderate clot: inc in RV/RA pressures, decrease in gas exchange, risk of some lung infarction Clot in a main pulmonary artery: does not equate to a pneumonectomy (which would be fine) because vasoactive factors leak out of the clot area 100 Giles Kisby GE Y1 Respiratory o - causing the remaining functional lung to vasoconstrict thereby giving pulmonary hypertension Situations when instantaneous inc in pressure occurs means RV is more likely to fail than if occurred slower allowing RV to hypertrophy Pulmonary hypertension: o Pulmonary hypertension (PH) is an increase of blood pressure in the pulmonary artery, pulmonary vein, or pulmonary capillaries, together known as the lung vasculature, leading to shortness of breath, dizziness, fainting, leg swelling and other symptoms. Pulmonary hypertension can be a severe disease with a markedly decreased exercise tolerance and heart failure o Normal ranges for PAPmean: Rest: <20 mmHg = good 20 25 mmHg = borderline >25 mmHg = threshold for “pulmonary hypertension” is exceeded Exercise: often as high as >30mmHg o Subtypes: Pulmonary venous hypertension Primary defect usually cardiac Elevated pulmonary capillary wedge pressure Pulmonary arterial hypertension PAH Primary defect in pulmonary arteries Elevated pulmonary vascular resistance (PVR o o Broad causes: physiological pathological Causes: [any can lead to RV failure] 1) Pulmonary arterial hypertension (primary defect in pulmonary arteries) -primary -related to collagen/vascular diseases, congenital heart disease, drugs, toxins, HIV, etc 2) Pulmonary venous hypertension (primary defect usually cardiac) -left sided atrial, ventricular or valvular disease 101 Giles Kisby GE Y1 Respiratory - 3) Pulmonary hypertension associated with disorders of the respiratory system and/or hypoxia When right ventricular failure develops in the setting of chronic lung disease and hypoxia, it is termed cor pulmonale [ie not due to left heart problems etc] 4) Pulmonary hypertension due to chronic thrombotic and/or embolic disease 5) Pulmonary hypertension associated with miscellaneous disorders Disease states overview: o - pneumonia o - pulmonary oedema o - pulmonary embolus o - pulmonary hypertension o - anatomical pulmonary shunts 102 Giles Kisby GE Y1 Respiratory Respiratory failure: - Lectures 17 and 18: Respiratory Failure I and II. Dr Umeer Waheed, Umeer.Waheed@imperial.nhs.uk, Dr Richard Stumpfl,Richard.Stumpfle@imperial.nhs.uk At the end of these lectures you should be able to o o o -a gradient in Type 1 and 2 Respiratory Failure o ory Distress syndrome o 29/11/13: Respiratory failure I: Dr Richard Stümpfle Los from slides: • 1) Understand how the pathological processes of respiratory failure interfere with normal respiratory physiology • 2) Explain how alterations in different aspects of respiratory physiology result in the different types of respiratory failure • 3) Following lectures 1 and 2, have a basic understanding of treatment principles of respiratory failure Notes: - Introduction o The reported incidence of ARF varies between around 78–149 per 100 000 people >15 years of age per year o Ninety-day mortality is close to 40% for ARF and 31–60% for ALI / ARDS o The majority of patients have ARF of pulmonary origin with pneumonia as the predominant diagnosis - Definition: o Respiratory system unable to maintain adequate gas exchange to satisfy metabolic demands, i.e. the demands for: Oxygenation Elimination of carbon dioxide 103 Giles Kisby GE Y1 Respiratory - Consequences o Hypoxaemia is inadequate oxygenation of blood o Anaerobic metabolism starts ~ when PaO2<4.5kPa o Leads to reduced cellular function, lactic acidosis & cell death o Different tissues have different tolerances o Reversible loss of function starts at: Cerebral tissue 1min Myocardial tissue 4mins Skeletal muscle 2hrs o Can lead to multiorgan failure and death - Classification: o (Type 1): Hypoxaemic [lack of O2 in blood]: Failure of gas exchange at alveolar level PaO2 <8.0 kPa Normal or low CO2 o (Type 2): Hypercapnic: Ventilatory failure PCO2 >6.0 kPa - Non-respiratory Functions of the lungs: o Reservoir of blood available for circulatory compensation o Filter for circulation: Thrombi, microaggregates etc. o Metabolic activity: Activation: Angiotensin I→II Inactivation: Noradrenaline Bradykinin 5 H-T: 5-Hydroxytryptophan Some prostaglandins o Immunological: IgA secretion into bronchial mucus - Oxygen Cascade: o Three key drops / losses in PO2: 1. Humidification and mixing with CO2 2. Diffusion from alveoli to capillaries 3. Physiological shunts 104 Giles Kisby GE Y1 Respiratory o o o PAO2 dependent on total alveolar pressure & partial pressures of other gases in alveolus: Alveolar Pressure=PAO2+PACO2+PAH2O+PAN2 Ie if CO2 conc greatly increases then will dilute O2 (other gases get pushed out of the alveoli) or if high altitude then amount of O2 present will fall Factors that can result in changes to PAO2 include: PACO2 Alveolar pressure FiO2 [fraction/percentage of oxygen participating in gas-exchange] Ventilation alveolar ventilation equation: the fundamental relationship of respiratory physiology describes the inverse relationship between alveolar ventilation and alveolar PCO2 (PACO2). The constant, K, equals 863 mm Hg for conditions of BTPS: means body temperature (310 K), ambient pressure (760 mm Hg), and gas saturated with water vapour. if CO2 production is constant, then PACO2 is determined by alveolar ventilation 105 Giles Kisby GE Y1 Respiratory o [nb knew that it would be a reciprocal relationship (y=1/x) rectangular hyperbola from the equation] Alveolar gas equation PAO2=PIO2-(PACO2/R) PAO2, PiO2 are the partial pressures of oxygen in alveolar and inspired gas R = respiratory quotient (Respiratory Exchange Ratio) = 0.8 usually Ie indicates some things that could be a means of giving hypoxia alveolar gas equation: is used to predict the alveolar PO2, based on the alveolar PCO2; or change in PAO2 that will occur for a given change in PACO2 [nb can use for a variety of locations as shown] The correction factor is small and usually is ignored. 106 Giles Kisby GE Y1 Respiratory - Carbon Dioxide Elimination: o CO2 has high diffusion coefficient & is very water soluble (cf. O2 is much less soluble, therefore Hb used) o CO2 elimination dependent on alveolar ventilation o Alveolar Ventilation=Respiratory Rate x (Tidal Volume-Dead Space) o Factors that can result in changes to PACO2 include: Respiratory Rate: if fever or seizure are metabolically more active so can generate more CO2 Tidal Volume V/Q matching o If carbon dioxide levels are high, the body assumes that oxygen levels are low, and accordingly, the brain's blood vessels dilate to assure sufficient blood flow and supply of oxygen. Conversely, low carbon dioxide levels cause the brain's blood vessels to constrict, resulting in reduced blood flow to the brain and lightheadedness. - Dead Space o Fraction of tidal volume that does not take part in gas exchange o Dead space is the volume of air which is inhaled that does not take part in the gas exchange, either because it (1) remains in the conducting airways, or (2) reaches alveoli that are not perfused or poorly perfused o Anatomical dead space relates to volume of conducting passages Mouth, trachea etc. 2.2ml/Kg body weight, or ⅓ tidal volume Measured by Fowler’s method (N2 washout) o Alveolar dead space relates to V/Q mismatch o Physiological dead space = Alveolar + Anatomical dead space - Ventilation o Ventilation results in renewal of the A-a gradient o Hypoventilation results in a rise in PaO2 & PaCO2 107 Giles Kisby - GE Y1 Respiratory Causes of Respiratory Failure o Hypoventilation (nb CO2 levels are more sensitive to hypoventilation than O2 levels) Brainstem Injury due to trauma, haemorrhage, infarction, hypoxia, infection Metabolic encephalopathy Drugs Spinal cord Trauma, tumour, transverse myelitis Nerve root injury Nerve Trauma Neuropathy Motor neuron disease Neuromuscular junction Myasthenia gravis Drugs Respiratory muscles Myopathy Atrophy Muscular dystrophy Fatigue Respiratory system Airway obstruction o Resistance is obstruction to air flow by conducting airways o Resistance is main contributor to respiratory work Reduced compliance (lung, pleural, chest wall) o Compliance relates to distensibility or ‘stretchiness’ Lung compliance o Chest wall compliance o Diffusion impairment Lungs provide 50-100m2 surface area for diffusion O2 diffuses from alveolus to capillary until partial pressures equal The diffusion is a passive process Equilibrium completed in ⅓ time of capillary blood flow (0.75s) [ie as mentioned at such healthy regions there is not an Aa gradient but elsewhere physiological shunts occur] Allows for compensation in higher cardiac output states Does not affect CO2 as more soluble Causes include: Pneumonectomy 108 Giles Kisby GE Y1 Respiratory o o Pulmonary fibrosis [the diffusion distance increases] Emphysema Pulmonary oedema Anatomical Shunting V/Q mismatch: OVR Aa gradient exists due to physiological shunting (a capillary at a healthy ventilated alveolus would become fully oxygenated); Aa gradient increases with age Perfusion: Determined largely by gravity Perfusion pressure highest at bases At apices perfusion pressure may fall below alveolar pressure Ventilation Varies according to position of alveoli on compliance curve [ie greater work of emphysema / fibrosis etc can lead to hypoventilation] Compliance greater at apex of lung (stretchier, less weight on them, ventilate better) o Means that force exerted on the capillaries (which are already poorly perfused at the apex) is greater so tend to be squashed flat Compliance lower at base of lung (less stretchy, more weight on them, ventilate worse) o CPAP allows greater utilisation of these alveoli in a healthy lung [will cause greater occlusion of capillaries at apexes of lungs giving deadspaces but will give increased ventilation at bottom of lung reducing the number of partial shunts occurring; many more alveoli at bases so OVR positive effect] Ideal ventilation occurs somewhere midway up the lungs where neither perfusion nor ventilation are too dominant but instead are balanced Alveolar Dead space Well ventilated alveoli but no perfusion Causes include: o Pulmonary embolus o Low cardiac output states o High intra-alveolar pressure Shunting Alveoli not ventilated but perfused Therefore blood not oxygenated Resulting hypoxia resistant to increases in FiO2 Hypoxic pulmonary vasoconstriction limits perfusion to poorly ventilated alveoli Commonest cause of hypoxia in critically ill 109 Giles Kisby GE Y1 Respiratory - Causes include: o Any cause of left to right intra-cardiac shunt [ie anastomoses exist where certain bronchial arteries drain to pulmonary veins] o Pneumonia o Pulmonary oedema/haemorrhage o Atelectasis [defined as the collapse or closure of the lung resulting in reduced or absent gas exchange] – increases with age; these areas are at base and fail to be ventilated Assessment: o History Presenting complaint Past medical history Medications Social history o Examination Signs of respiratory compensation Tachypnoea [the condition of rapid breathing] Use of accessory muscles Nasal flaring Intercostal, suprasternal or supraclavicular recession Increased sympathetic tone Tachycardia [he condition of rapid breathing] Hypertension Sweating Haemoglobin desaturation Cyanosis [the appearance of a blue or purple coloration of the skin or mucous membranes due to the tissues near the skin surface having low oxygen saturation] Hypercapnoea Respiratory flap [Asterixis (also called the flapping tremor, or liver flap) is a tremor of the hand when the wrist is extended, sometimes said to resemble a bird flapping its wings] Hypertension Coma End-organ hypoxia Altered mental status Bradycardia/hypotension ‘A’ is for airway ‘B’ is for breathing ‘C’ is for circulation 110 Giles Kisby GE Y1 Respiratory o o - Summary o o o o ‘D’ is for disability Investigations Arterial blood gases Lung function tests Chest X-ray Computed tomography scan (CT scan) Microbiological tests Management Oxygen Non-invasive ventilation Invasive ventilation Treat underlying cause Respiratory failure results in hypoxia & hypercapnoea Acute respiratory failure associated with high mortality Causes related to disruption in physiology Simple approach to assessment & management 111 Giles Kisby GE Y1 Respiratory 29/11/13: Respiratory failure and treatment: Umeer Waheed Los from slides: • • • • • Differentiate between Type 1 and 2 Respiratory Failure Outline the management of Type 1 and 2 Respiratory Failure Describe the importance of A-a gradient in Type 1 and 2 Respiratory Failure Describe the pathophysiology of Acute Respiratory Distress syndrome Outline the treatment modalities for Acute Respiratory Distress syndrome Notes: - General details: o All acute lung conditions with the exception of obstructive lung disease that require active therapy. o Not a specific disease, but a reaction to an underlying condition o The underlying condition strongly influences prognosis. Lung Cancer Pneumonia o Incidence of people >15 years of age per year admitted to Hospital 78 /100000 90 day mortality 40% ARF ( Acute Respiratory Failure) PaO2 < 8kpa ALI( Acute Lung Injury) PaO2/FiO2* <300 mmHg (40 kPa) ARDS ( Acute respiratory Distress Syndrome) PaO2/FiO2 <200 mmHg (26.7 kPa) ARF pulmonary origin 52% Pneumonia 23% o Acute respiratory failure occurs when: Pulmonary system is no longer able to meet the metabolic demands of the body Failure of oxygen transfer across the alveoli Failure of oxygen transport to the tissues Removal of CO2 from the blood / alveolus to the environment o Hypoxaemic respiratory failure Type I PaO2 <8kPa when breathing room air o Hypercapnic respiratory failure Type II PaCO2 >6.7 Kpa o Ventilatory Capacity (VC) V Ventilatory Demand (VD) VC maximal spontaneous ventilation that can be maintained without fatgue 112 Giles Kisby GE Y1 Respiratory o o o - Type I o o - VD Spontaneous minute ventilation that results in a stable PaCO2 Usually VC > VD Respiratory failure may result from reduced VC or increase VD or both. Acute Life threatening derangements in ABG’s and acid base status Develops in mins / hours , pH <7.3 Chronic Usually less dramatic and may not be readily apparent, unless associated with acute deterioration Develops in days allowing time for renal compensation, increase in [HCO3} and normal pH NB acute on chronic may occur: prob will give an even worse prognosis than normal acute resp failure Type II o o Normocapnic / Hypocapnic, hypoxaemia [ie ventilation but still not enough gas exchange for O2 needs: hypoxia without hypercapnia – ie some exchange occurs and is enough for CO2 removal] ARDS (see below) Severe Pneumonia Pulmonary embolus ie Pulmonary Embolism Pulmonary odema Emphysema [Acute exacerbations of chronic obstructive pulmonary disease (AECOPD)] Asthma (ie but not as severe as for type II: asthma first gives type I and then when more severe then gives type II) Pulmonary haemorrhage / Trauma Foreign body (ie but not as severe as for type II) Ob. Sleep Apnoea (ie not severe enough to give type II) Interstitial Lung disease Sickle cell crisis Pneumothorax Pleural Effusion Hypercapnic, Hypoxaemia inadequate ventilation; both oxygen and carbon dioxide are affected CNS: Neuromuscular disease Respiratory centre depression CVA, Drugs, Infection, Tumours, Trauma head and neck Alveolar hypoventilation [a hypoventilation syndrome] Nerve injuries Drug OD, CVA, = cerebrovascular accident = stroke 113 Giles Kisby GE Y1 Respiratory tumor, Central hypovent’ (Ondine's curse), hypothyroid Muscle cell: Guillain Barre, [is an acute polyneuropathy, a disorder affecting the peripheral nervous system] Myaesthenia Gravis, Post polio syndrome, [muscular atrophy (decreased muscle mass) etc] Botulism, MND Spinal shock Myopathies Peripheral airways COPD, Asthma Musculoskeleto-pleural Kyphoscoliosis, Chest wall, pleural problem Other Foreign Body, Laryngeal edema, subglottic stenosis, Obstructing tumor - Refractory Hypoxaemia o Presence of a low PaO2 despite increasing O2 : giving them oxygen doesn’t solve the problem o implies more to the hypoxaemia than a simple diffusion problem at alv-cap interface. o Problems with perfusion likely occurring: V/Q matching (& HPV) - ARDS I: Acute respiratory distress syndrome: adults [also known as adult respiratory distress syndrome] o Is TYPE 1 respiratory failure o defined by oxygenation: arterial oxygen tension / fractional inspired oxygen (Pa02/Fi02) < 200mmHg (27 kPa) for ARDS o spectrum of acute lung injury: [mild - acute lung injury (ALI) and] severe - acute respiratory distress syndrome (ARDS) o very high mortality if in conjunction with failure of other organs o refractory hypoxaemia o CXR - bilateral diffuse infiltrates o absence of cardiogenic pulmonary oedema o Direct vs indirect causes: [ie there are many different causes of ARDS] e.g. pneumonia vs acute pancreatitis, e.g. pulmonary contusion vs abdominal sepsis 114 Giles Kisby GE Y1 Respiratory o o o o - Pathophysiology - High permeability pulmonary oedema - V/Q mismatch *loss of HPV *atelectasis / volutrauma Mix of diseased lung (gravity dependent) and normal lung Traits: Cytokines that are present due to whatever the specific cause in that patient is will cause plasma leak from post capillary venules inc alv-cap distance dec diffusion Deregulation of vascular reactivity loss of HPV Intrapulmonary microvascular shunt [shunting is mainly happening at the lower, well perfused lung regions where the fluid collects] Dead space ventilation in other areas [due to higher lung regions being ventilated well but poor perfusion (normally there areas not required so would be fine but fluid elsewhere means they need to be used but HPV dysregulation means are not being used either] V/Q mismatching [due to the shunting] Refractory hypoxaemia Gravity results in the region of the lung that is lower being fluid filled; however this is strong overlap with the areas of the lung that have the best blood supply so put patient on front where fewer well perfused lung units (even when have turned over in this way because there are fewer lung units that side) thereby symptoms can be eased Disparities within the lungs: normal lung better compliance risk of volutrauma due to greater pressures [patient physiological response / CPAP / ventilation increases openings and closings of aerated units giving high shear stress Dependent lung reduced compliance [think of compliance of a wet sponge] greater pressure support to recruit collapsed alveoli [ie dependant lung ~ lower lung regions so here CPAP can act to allow recruitment of collapsed alveoli Monitoring o Respiratory compensation Tachypnoea Use of accessory muscles Recession Nasal flaring 115 Giles Kisby GE Y1 Respiratory o o o Sympathetic stimulation (adrenaline gets secreted, etc which gives bronchodilation but also other effects) Tachycardia Hypertension Palor Sweating Cool peripheries Tissue hypoxia [ie can lead to failure of other organs] Altered mental state Hypotension Bradycardia Renal failure Haemaglobin desaturation Cyanosis - Pulse Oximetry o Pulse oximetry is a non-invasive method allowing the monitoring of the oxygenation of a patient's hemoglobin. o Light at red (660nm) and infrared (940nm) wavelengths is passed sequentially through the patient to a photodetector o The changing absorbance at each of the two wavelengths is measured, allowing determination of the absorbances due to the pulsing arterial blood alone o Based upon the ratio of changing absorbance of the red and infrared light caused by the difference in color between oxygen-bound (bright red) and oxygenunbound (dark red or blue, in severe cases) blood hemoglobin, a measure of oxygenation (the percentage of hemoglobin molecules bound with oxygen molecules) can be made. o Sources of error Poor peripheral perfusion Poorly adherent probe False nails/nail varnish Lipaemia Bright ambient light Excesive motion Carboxy/Met haemaglobin o It is also important to be aware that the saturation may remain normal in the face of significantly impaired ventilation, particularly if the patient is receiving oxygen therapy - Treatment o Supportive Oxygen Therapy 116 Giles Kisby GE Y1 Respiratory o Continuous positive airways pressure CPAP continuous positive airway pressure reduces shunt by recruiting partially collapsed alveoli at base of lung (ie prob recruits more alveoli than the number of alveoli that become impinged with regard to their bloodflow due to the inc in pressure) maintains patency of more alveoli at end-expiration (PEEP) useful post surgery - retained secretions due to pain-induced shallow breaths and basal atelectasis; as an adjunct to analgesia and physiotherapy. reduces work of breathing: as below it shifts patients position on the curve right by giving an initial contribution to pressure o Mechanical Ventilation When deciding if should do this must consider: Severity of respiratory failure Cardiopulmonary reserve Adequacy of compensation Expected speed of response Risks of mechanical ventilation Non respiratory indication for intubation Varients: Frequency Oscillatory Ventilation o Shallow tidal volumes used Extracorporeal Membrane Support o Blood oxygenated externally 28/11/13: Exercise: Dr Luke Howard 117 Giles Kisby GE Y1 Respiratory Los from booklet: Lecture 15: Exercise Physiology. (Dr Luke Howard l.howard@imperial.ac.uk) At the end of this lecture you will appreciate and understand ion Notes: - Basic exercise metabolism: o CO2 levels are responded to by heart and lungs to give sufficient clearance and because one O2 is required per 0.8 CO2 produced this it is relatively intuitive that the heart and lung activities will also be sufficiently matched to the metabolic demands of the body in this manner However upon anaerobic metabolism at peak exercise the amount of O2 being used is nolonger increasing but amount of CO2 formed is still increasing so signals for inc RR and subject in fact begins to breath higher than their O2 needs CO2 signals for resp change via chemoreceptors (see prev notes) Heart rate changes are due to several inputs [presumably then all signal via the sympathetic system]: Your brainstem receives signals from many parts of your body that help it determine how much to speed up heart rate when you exercise. Some signals come from the motor cortex, a part of your brain that coordinates movement. Other signals come from receptors in your muscles and joints that sense movement as you start exercising. The hypothalamus is a part of your brain that controls body temperature. When you exercise, your body temperature increases, causing the hypothalamus to signal the brainstem to further increase your heart rate. o o o o o Muscle contraction requires energy for contraction in the form of phosphate, “delivered by ATP” Sources of phosphate: Anaerobic metabolism, incl. Creatine phosphate Aerobic metabolism Largest source is aerobic Cardiac output needs to increase by 5-6 l/min per l/min oxygen consumption [at rest CO ~5lmin-1 but can increase to ~25lmin-1 upon exercise] If it fails, anaerobic metabolism required 118 Giles Kisby GE Y1 Respiratory o o o o o o o o o Total body H+ is 3.4 micromoles H+ generated at walking speed is 40,000 micromoles per minute from CO2 production Cardiovascular system must remove CO2 to lungs where it is eliminated in proportion to rate of delivery Small errors lead to large changes in pH but in fact remains very constant during exercise to ensure disruption to enzymatic systems does not occur At end of maximal exercise high lactic acid production means that bicarbonate reserves must be used up to be combined with H+ to give CO2 which can be breathed out – when bicarbonate reserves are depleted pH will start to fall so will be forced to stop exercising Ie presumably the rate of CO2 production will level off / fall as are no longer generating CO2 from acids CO2 + H20 ↔ H2CO3 ↔ H+ + HCO3Very tight relationship between ventilation (Lmin-1) and CO2 production The relationship of ventilation to carbon dioxide production relates to the efficiency of the lungs [ie what gradient this tight line takes] Is a good measure of VQ mismatching: eg if pulmonary embolism / pneumonectony then will require a greater ventilation rate for a given amount of CO2 clearance Relationship may be lost if eg hyperventilation at rest Sources of CO2 Output during Exercise 1) Aerobic substrate catabolism 2) Bicarbonate buffering from H+ produced alongside lactate 3) Pulmonary hyperventilation due to acidosis [ie breathing faster gives greater clearance (in short term) despite not actually making any more CO2 in the body] – occurs at the end of exercise RER: Respiratory exchange ratio: The ratio between the amount of CO2 produced and O2 consumed in one breath (determined from comparing exhaled gasses to room air) is the respiratory exchange ratio (RER). In one breath, humans normally breathe in more molecules of oxygen (O2) than they breathe out molecules of carbon dioxide (CO2). RER is about 0.8 at rest with a modern diet but tends to exceed 1 with intense exercise (see below) CO2 production / O2 consumption = ~0.7 (ie less than 1) if metabolising fats as the ratio in its metabolism is 16:23 1 palmitate + 23 O2 → 16 CO2 +129 ATP If anaerobic metabolism of glucose/glycogen then CO2 production / O2 consumption > 1 as lactate gives CO2 without O2 consumption glucose → 3 ATP + 2 H+ + 2 Lactate 2 H+ + HCO3- → 2 CO2 [anaerobic threshold has been surpassed: ie can see when threshold passed by the sudden increase in the CO2 production : O2 consumption ratio] Above anaerobic threshold, CO2 production exceeds O2 consumption. 119 Giles Kisby GE Y1 Respiratory o - DO NOT HAVE TO BE ABLE TO INTERPRET EXERCISE TEST FOR THE EXAM; ONLY UNDERSTAND THE PHYSIOLOGY ETC o o - Seen as clear upward deflection on CO2 production vs O2 consumption graph. Higher RER on exercise indicates how far past anaerobic threshold have gone As ventilation is coupled to CO2 production, ventilation rises disproportionately to O2 consumption CO2 production per ATP generated rises massively compared to either aerobic metabolism type (fat / glu) Lactate itself is converted back to glucose in the liver This process requires O2: “oxygen debt” If mainly metabolising glucose (/glycogen etc) then (assuming aerobic) CO2 production / O2 consumption = ~1 because one O2 used per CO” made 1 glucose + 6 O2 → 6 CO2 +37 ATP [is actually 38ATP] For incremental work: Aerobic metabolism more efficient than anaerobic [more ATP per O2] and produces less CO2 per ATP generated Slightly more ATP per O2 than achieved with fat metabolism At exercise may be breathing 30/35 breaths per min max [and each exhale is therefore effectively an FEV1]; so maximum breathing capacity (=maximum voluntary ventilation) (he called it max ventilatory capacity) will be ~30*FEV1 = ~200Lmin-1 for me Ie max breathing rate and FEV1 both variables that determine the max O2 supply to lungs that an individual can achieve Good rule of thumb is 220 – [age] to give predicted max HR of a person O2: o o Factors affecting amount of O2 consumption upon exercise: Muscle bulk Effort & neuromuscular coupling Capillary & mitochondrial density Cardiac output = Stroke vol. x HR Haemoglobin Oxygen saturation V/Q matching FEV1 Respiratory muscle function NB also “heart rate reserve” and “breathing / ventilation reserve” are key limiters to amount of O2 that can be supplied on exertion 120 Giles Kisby GE Y1 Respiratory o o o o o o o o - VO2 = rate of delivery of delivery of oxygen to tissues CaO2 and CvO2 = conc of O2 at arteries and veins Fick equation VO2 = CO x (CaO2 – CvO2) VO2 = SV x HR x 1.34 x Hb x (SaO2 – SvO2) 1.34 is just a constant Nb Sv increases before HR; both OVR inc during exercise (SaO2 – SvO2) is usually about the same for all healthy people At peak exercise: CaO2 21 ml/100ml CvO2 5 ml/100ml “oxygen pulse” = VO2 / HR VO2 / HR α SV x (SaO2 – SvO2) VO2 max: value relative to body weight is what indicated fitness the maximum capacity of an individual's body to transport and use oxygen during incremental exercise, which reflects the physical fitness of the individual. VO2 max is expressed either as an absolute rate in litres of oxygen per minute (L/min) or as a relative rate in millilitres of oxygen per kilogram of bodyweight per minute (i.e., mL/(kg·min)). CO2: o o o VCO2 = rate of CO2 production: Ventilation increases in proportion to CO2 production Vd/Vt = deadspace as a fraction of total lung volume: The more deadspace in your lung, the more you have to breathe to clear CO2 Eg emboli etc Decreases upon exercise as tidal volume increases as deadspace of trachea etc doesn’t change while new lung recruited Ventilation rate, VE = VCO2 * (863 / (PaCO2 * (1-[Vd/Vt])) In fact VE might be ventilatory efficiency (as in the efficiency of CO2 removal from the body) but the same proportional relationships will exist Ie with inc ventilation first efficiency rises as fractional deadspace falls but eventually hyperventilate and if PaCO2 falls then would give decrease in efficiency VE VCO2 . o o 863 PaCO2 .(1 VD VT ) PaCO2: NB high ventilation rate corresponds to low PaCO2 thus the two values are inversely related Nb patient with heart failure will have a high VE / VCO2 121 Giles Kisby GE Y1 Respiratory - HR and SV and dilation influences: o Heart rate Autonomic nervous system Drop in vagal tone Rise in sympathetic stimulation Activated by cortical and muscle ergoreceptor mechanisms (detail was touched on above) o Stroke volume Increased venous return from contracting muscles Decreased intrathoracic pressure with deep inspiration “sucking blood in to the lungs” Sympathetic response (increasing inotropy: inc force of muscular contractions) Reaches peak at 50% of peak VO2 [ie then levels off] o Preferential dilatation of active vascular beds Autonomic nervous system Local factors H+ CO2 K+ Omsolarity Nitric oxide Adenosine Temperature PO2 - Ventilation change influences: o Comfortable tidal volume is approx. half of vital capacity o Three stages of ventilator change: Initial sharp rise: Cortical: direct signalling from brain Ergoreceptors: afferents sensitive to muscle contraction Cardiodynamic: SV increases [somehow this gives inc ventilation; don’t worry about mech; 'cardiodynamic hyperpnoea' considers a possible effect of increasing cardiac output on ventilation] Parabolic rise CO2 inc production rate signalling back for inc ventilation Flattening off Maximum ventilation reached 122 Giles Kisby - GE Y1 Respiratory Exercise: o Excellent matching occurs between O2 consumption, CO2 production, and the ventilation rate. o mean values for arterial PO2 and PCO2 do not change during exercise [thought that very slight oscillations are enough to give the necessary signalling to ventilation rate] [nb but hyperventilation in late exercise as anaerobic gives HCO3production] o The arterial pH may decrease, however, during strenuous exercise because the exercising muscle produces lactic acid [ie if HCO3- compensation incomplete] o decrease in the physiologic dead space: CO inc pulmonary blood flow increases perfusion of more pulmonary capillary beds: pulmonary blood flow becomes more evenly distributed throughout the lungs, and the V/Q ratio becomes more “even,” There is a decrease in pulmonary resistance associated with perfusion of more pulmonary capillary beds [useful in catering for the inc CO] o The PCO2 of mixed venous blood increases during exercise o During exercise, the O2-hemoglobin dissociation curve shifts to the right 123 Giles Kisby GE Y1 Respiratory 124 Giles Kisby GE Y1 Respiratory [ONLINE SELF LEARNING FROM SLIDES]: Altitude and air travel: Dr Robina Coker - Read pdf in folder if possible as good detail on the physica, physiology and pathophysiology Los from booklet: tests Notes: - Why is air travel important o 2002 414 commercial flight diversions, each costing £100,000; Respiratory conditions are the 3rd commonest cause o 2005 15,550 in-flight medical emergencies o 2006 17,300 in-flight medical emergencies (30% of airlines) o 2006 89 in-flight deaths o 2007 77 in-flight deaths (1 in 5.7m passengers) - Altitude and hypoxaemia: o O2 concentration of dry air at sea level is 20.9% (~21%) o Inhaled air is warmed and saturated with water vapour. Partial pressure of water vapour at 37oC is 47mmHg o PIO2 at sea level = 0.21x (760-47) = 149 mmHg [At sea level, the atmospheric pressure is 760mmHg] o PIO2 at 3,450m (Jungfrau Joch train station) = 0.21 x (497-47) = 94 mmHg ie 2/3 that at sea level [ie PIO2 drops significantly with altitude]: 125 Giles Kisby - GE Y1 Respiratory Humidified gas expansion o gas expansion for saturated gas is greater than for dry gas: o relative expansion=(initial pressure of gas in cavity at sea level – 47 mmHg) for humidified gas (final pressure of gas in cavity – 47 mmHg) [47 mmHg = pressure of water vapour] Eg. (760 - 47) / 566 - 47) = 713/519 = 1.37 Vs for dry gas = 760/566 = 1.34) volume of saturated gas in a non-communicating bulla rises by 37% on ascent to 2438 m (8,000 ft) Cabin Humidity and Dehydration To achieve pressurisation in the cabin they take ambient air and compress it. Since the gas heats up in this process, it must subsequently be cooled. The resulting air is of low humidity which helps avoid stress on the plane upon change in altitude and reduces the threat of corrosion but can cause skin dryness and discomfort in the eyes, mouth, and nostrils and favour dehydration. The air is therefore recycled repeatedly to rise humidity but it remains low: usually less than 10% to 20% humidity [in the home is normally over 30%]. o o o - Respiratory Indications for Clinical Evaluation Prior to Air Travel: [ie anything that may indicate predisposition for or sensitivity to low O2 saturation levels]: o Moderate to severe chronic obstructive pulmonary disease o Persistent severe asthma 126 Giles Kisby GE Y1 Respiratory o o o o o o o o o - Clinical pre-flight assessment o Various possible methods: o 50 metre walk [In such a test, the aim is to verify that the patient is capable of walking 50 m without limitation due to dyspnea] o FEV1 o Oximetry o Regression equations o Hypoxic challenge test (HCT) o Hypobaric chamber testing o o - Severe restrictive disease (including diseases of the chest wall and respiratory muscles), especially with hypoxemia or hypercapnia Cystic fibrosis History of intolerance of air travel due to respiratory symptoms (dyspnea, chest pain, confusion, or syncope) Comorbid conditions that are worsened by hypoxemia (cerebrovascular disease, ischemic heart disease, heart failure) Pulmonary tuberculosis Patients from areas with recent local outbreaks of severe acute respiratory syndrome Recent pneumothorax Risk or previous episode of venous thromboembolic disease Prior use of oxygen therapy or ventilatory support Predictive role of FEV1 and oximetry currently unclear Medical history: special attention should be paid to recognizing all cardiorespiratory disease, with particular interest in comorbidity that could be worsened with hypoxemia (cerebrovascular disease, ischemic heart disease, heart failure). Air travel with oxygen o Supplementary oxygen is recommended during air travel for patients who have an estimated in-flight PaO2 of less then 50 mm Hg o In COPD [see COPD detail later below], O2 at 2L/min improves but does not fully correct hypoxaemia at 2438 m; O2 at 4L/min over-corrects o Aircraft delivery usually 2 or 4L/min from cylinder: ie are not allowed to bring your own oxygen on the flight (due to safety/terrorism concerns) o Nasal prongs recommended (for access of the oxygen from the cylinder to the body) o Check regulations and charges with airlines 127 Giles Kisby GE Y1 Respiratory - Key learning points o With increasing altitude, barometric pressure falls o This causes a progressive fall in the partial pressures of inspired and arterial oxygen o It also leads to an increase in gas volumes (especially if the gas is saturated) o Hypoxaemia triggers a series of adaptive physiological responses, termed acclimatisation o Failure of acclimatisation can lead to altitude related illnesses o Clinical assessment of those with lung disease prior to air travel or (less commonly) high altitude exposure may require evaluation of cardio-respiratory status, hypoxic challenge and/or hypobaric chamber testing - Other info from internet o Hypobaric = Below normal pressure o Commercial aircraft generally fly at an altitude of around 11 000 to 12 200 m: Flights occur in the troposphere: extends from sea level to 9144 m (30 000 feet) at the poles and to 18 288 m (60 000 feet) at the equator o The pressurization system used by commercial aircraft is known as isobaric.27 Initially, as the aircraft climbs in altitude, it maintains the same ambient pressure as its environment, and then, from a certain altitude, it maintains a constant (isobaric) pressure, irrespective of changes in altitude. o Aircraft pressure is not maintained at that of sea level but rather at an intermediate pressure; that pressure depends on the type of aircraft but is usually approximately equivalent to that of an altitude of 2400 m; At that altitude, the atmospheric oxygen tension is equivalent to breathing 15.1% oxygen at sea level. o o o o In healthy subjects, this can represent a reduction in PaO2 from 98 to 55 mm Hg, which is usually well tolerated and does not produce symptoms However, in patients with chronic respiratory diseases and some degree of baseline hypoxemia, the reduction in PiO2 during the flight can cause more marked reductions in oxyhemoglobin saturation Acute exposure to a hypobaric environment triggers hyperventilation, which is essentially induced by stimulation of peripheral chemoreceptors and is usually mediated by an increase in tidal volume. It also generates an increase in cardiac output to compensate for the residual systemic hypoxia. This increase is mainly mediated by tachycardia and is usually proportional to the drop in oxygen saturation. The increased pulmonary perfusion caused by the rise in cardiac output is associated with hypoxic vasoconstriction [ie due to low oxygen levels] of the pulmonary artery and increased systolic pulmonary pressure. As a consequence of the increase in pulmonary vascular resistance, there is a redistribution of pulmonary blood flow and an increase in perfusion of certain areas of the lungs 128 Giles Kisby GE Y1 Respiratory o o o compared with the situation at sea level [ie high perfusion due to vasoconstriction elsewhere and due to high CO; therefore prob a risk of pulmonary edema at these highly perfused areas]. Altitude is also associated with limitation of oxygen diffusion from the atmosphere into the pulmonary capillaries as a consequence of the interaction of various factors. Both the reduced PiO2 and the reduction in affinity of hemoglobin for oxygen in conditions of low PaO2 lead to a more marked drop in the oxygen content of the pulmonary capillaries than at sea level. Finally, there is a shortened transit time of blood through the pulmonary capillaries due to the tachycardia caused by the altitude and this limits the time available to establish an adequate oxygen equilibrium. The net result is an increase in the alveolar–arterial oxygen difference [increase in Aa gradient]. Exercise and hypobaric hypoxia: In addition, the oxyhemoglobin saturation is significantly reduced during physical exercise in a hypobaric environment. Exercise at high altitudes also increases the alveolar–arterial oxygen difference in subjects who normally reside at sea level, while it does not affect those native to high altitudes. Studies performed using the multiple inert gas elimination technique have shown that hypobaric hypoxia is associated with a greater heterogeneity in the ventilation–perfusion ratio and a limitation of diffusion that together worsen hypoxemia as exercise intensity increases. Limited diffusion secondary to reduced PiO2 appears to exert the greatest influence on blood gas alterations during exercise in a hypobaric environment. Additionally, the interstitial edema caused by extravasation of fluids into the extravascular space appears to potentiate the ventilation–perfusion imbalance. The changes described have few consequences in healthy subjects, who might only note a slight increase in tidal volume and heart rate. However, hypobaric hypoxia represents a risk for some patients with chronic respiratory disease, in whom it can aggravate pre-existing hypoxemia and favour the development of cardiovascular complications. In fact, it is recognized that hypoxia reduces the ischemic threshold in men with exercise induced ischemic heart disease as well as favouring some atrial arrhythmias and being associated with ectopic ventricular beats as a result of increased sympathetic activity Expansion of trapped gases: Common body areas with gases which will expand: Ears Paranasal sinuses Barodontalgia [dental pain] GIT [gastrointestinal tract usually contains some quantity of gas, and consequently, gastrointestinal discomfort is common during air travel] 129 Giles Kisby GE Y1 Respiratory o - Lungs [eg apical bullae can burst during ascent and cause a pneumothorax] Given that the gas in the body cavities is saturated with water vapour, the expansion caused by increased altitude is greater than that calculated according to Boyle’s law. The problem is much more severe in patients with chronic obstructive pulmonary disease (COPD), since those patients usually have regions of emphysema that are poorly connected with the exterior or separated from it and can cause rupture and pneumothorax, in addition to the problems generated by hypoxia. Diving: it is recommended that individuals do not fly within 24 hours following scuba diving Dissolved nitrogen can accumulate in the tissues (residual nitrogen) during scuba diving, particularly when diving is deep and repeated. During ascent, that nitrogen may be released and give rise to symptoms of decompression HIGH ALTITUDE: o one of several causes of hypoxemia o Eg: at 18,000 feet, PO2 = 70 mm Hg ([380 mm Hg - 47 mm Hg] x 0.21 = 70 mm Hg o Despite severe reductions in the PO2 of both inspired and alveolar air, it is possible to live at high altitudes if the following adaptive responses occur: Hyperventilation: if alveolar PO2 is less than 60 mm Hg respiratory alkalosis [can be treated with carbonic anhydrase inhibitors (e.g., acetazolamide)] dec ventilation renal compensation continued hyperventilation Polycythemia increase in red blood cell concentration O2-carrying capacity is increased,which increases the total O2 content of blood in spite of arterial PO2 being decreased. disadvantageous in terms of blood viscosity inc resistance dec flow stimulus for polycythemia is hypoxemia, which increases the synthesis of erythropoietin in the kidney. Erythropoietin acts on bone marrow to stimulate red blood cell production. increased synthesis of 2,3-DPG by red blood cells advantageous in tissues but not in lungs 130 Giles Kisby GE Y1 Respiratory o - Pulmonary Vasoconstriction pulmonary arterial pressure also must increase The right ventricle must pump against this higher pulmonary arterial pressure and may hypertrophy in response to the increased afterload. Acute Altitude Sickness The initial phase of ascent to high altitude is associated with a constellation of complaints, including headache, fatigue, dizziness, nausea, palpitations, and insomnia. The symptoms are attributable to the initial hypoxia and respiratory alkalosis, which abate when the adaptive responses are established. HYPOXEMIA o Hypoxemia is defined as a decrease in arterial PO2 o Aa gradient may exist in some cases 131 Giles Kisby GE Y1 Respiratory o o Supplemental O2 amay or may not be useful Causes: - High altitude Hypoventilation Diffusion defect V/Q defect Right to left shunt HYPOXIA: o Hypoxia is decreased O2 delivery to the tissues o Since O2 delivery is the product of cardiac output and O2 content of blood, hypoxia is caused by decreased cardiac output (blood flow) or decreased O2 content of blood. o Causes: Hypoxemia (due to any cause) is a major cause of hypoxia Dec CO Anaemia CO poisoning Cyanide poisoning [interferes with O2 utilization at tissue] 132 Giles Kisby GE Y1 Respiratory 133 Giles Kisby GE Y1 Respiratory 13/12/13: Lung cancer: Dr Shovlin [this lecture was actually given by someone else who didn’t put their slides up: will largely work from claire’s slides (largely because question bank q’s will prob have been written by her)] Los (from booklet): summarise the different cell types and function within the lung moking in different individuals Notes: - Lung cancer: o 4th most common cause of death in Uk o 5yr survival = 10% o Surgery is the only curative treatment But even then may have metastasised, etc o Resection not usually possible [lobectomy/pneumonectomy] Disease likely to have spread significantly through the lung Resection would reduce cardiopulmonary reserve Especially cannot resect if is at carina [unless pneumonectomy] o Symptoms: Cancer general symptoms: Night sweats Weight loss Fatigue Lung cancer: SOB Haemoptysis Local invasion Persistent / unexplained: chest/shoulder pain, hoarse voice, cough, dyspnoea Distant spread (metastases) Bone, brain, liver, adrenals, skin etc effects paraneoplastic syndrome: is a disease or symptom that is the consequence of the presence of cancer in the body, but is not due to the local presence of cancer cells.[1] These phenomena are mediated by humoral factors (by hormones or cytokines) excreted by tumor cells or by an immune response against the tumor. 134 Giles Kisby GE Y1 Respiratory o o o o Clubbing, malaise, weight loss Eg ADH production: causes low sodium – life threatening Eg PTH production: causes high Ca – life threatening The primary metastatic spread is by the lymphatics (not the blood) Metastasis is actually surprisingly uncommon Specific organs seem to be affected: Seed and soil hypothesis o The "seed and soil" hypothesis states that specific organs harbor metastases from one type of cancer by stimulating their growth better than other types of cancer. This interaction is dynamic and reciprocal, since cancer cells modify the environment they encounter. to diagnose: X ray Blood tests Lung function tests If cancer: CT Cytology of cells in sputum to identify type Histology of a biopsy Types of (primary) lung cancer: Small cell carcinoma [20% of cases] Closely packed neuroendocrine cells Rapidly spreads and metastasises The most aggressive type of lung cancer Treat with cisplatin or atoposide; ie chemotherapy [most of these patients respond but still poor survival] Non small cell carcinoma: [80% of cases] -Relitively chemoresistant [ie chemotherapy fails to gain response; any chemoresistant cells will be selected for so will relapse and then chemotherapy will have no effect second time] -most patients offered radiotherapy instead Squamous cell carcinoma [1/3] o Large pink tumour cells o Slow growing, late metastases o Necrosis in center of the tumour occurs Adenocarcinoma: [1/3] o Tumour cells from glandular tissues o May produce mucin Large cell anaplastic carcinoma [1/5] o Undifferentiated [Anaplasia refers to a reversion of differentiation in cells] 135 Giles Kisby GE Y1 Respiratory o Rarely: Mesothelioma: o Mesothelioma (or, more precisely, malignant mesothelioma) is a rare form of cancer that develops from cells of the mesothelium, the protective lining that covers many of the internal organs of the body. Mesothelioma is most commonly caused by exposure to asbestos.[1] The most common anatomical site for mesothelioma is the pleura (the outer lining of the lungs and internal chest wall) Management: Early diagnosis Early surgery where appropriate Chemotherapy alone or as adjunct to surgery Transplant Palliative care - Smoking and lung cancer o 85% of lung cancer occurs in smokers o Linear relationship of no. cigarettes smoked and lung cancer incidence o Mechanism is via inducing DNA damage At least 60 components of cigarettes are carcinogens P53 mutated in ~80% lung cancers [thought due to the carcinogens] P53 has key role in cell cycle arrest / apop Clara cell metabolism plays key role in activating the DNA damaging ability of many of the chemicals: both phase I and phase II enzyme action can lead to active metabolites eg from polycystic aromatic hydrocarbon precursors o DNA adduct is a piece of DNA covalently bonded to a (cancer-causing) chemical: are biomarkers of carcinogen exposure Increases with no. cigarettes smoked per day In smokers the adducts preferentially form at the codons most commonly known to mutate in p53 [implies direct smoking cause of the mutations] o Cigarettes have been made more healthy through time - Smoking and genetics: o A positive family history increases adjusted risk by 2/3 times Thought due to both shared genetic and shared env o Epigenetics may be part of the reason for heritability o EGFR-TK1 is involved in mitosis signalling via EGF signalling [ie is referring to the receptor] Inhibitors to the receptor can be used in treatment Better response if non smoker as is more likely to be a genetic problem of this pathway than just p53 dysfunction as is common in smokers 136 Giles Kisby GE Y1 Respiratory 137 Giles Kisby GE Y1 Respiratory 13/12/13: Hypoxia: Dr R. Davies Dr Sholvin [this lecture was the colossal disaster one and she hasn’t put any slides up; the Los are for dr shovlins but the corresponding lec cannot be found online] Los (from booklet) [but are for Dr shovlins lec which isn’t online either]: Lecture 12: Hypoxia (Dr Claire Shovlin, c.shovlin@imperial.ac.uk) At the end of this lecture you should be able to: (i.e. the O2 and CO2 dissociation curves), and factors affecting these curves with particular reference to oxygen uptake in the lung and the downloading of oxygen in the tissues. delivery to tissues and oxygen consumption; and the development of tissue hypoxia when delivery fails to meet demand with onset of anaerobic metabolism (lactic acid production) igh altitude There is an accompanying computer aided learning quiz which you can run through with Dr Shovlin which will assist your understanding of: rventilation, distinguishing hyperventilation from the „hyperpnoea‟ of exercise. -pulmonary capillary PO2 and PCO2. Explain the consequences of this for systemic arterial PO2 and PCO2. l) a reduction in Hb concentration in the blood (anaemia) affects PaO2, PaCO2 and oxygen content. -enriched gas mixture in correcting any abnormalities associated with anaemia. affects PaO2, PaCO2 and oxygen content, explaining the effectiveness (or lack of it) of breathing an oxygen-enriched gas mixture in correcting any abnormalities associated with hypoventilation. NB: lood Gases Notes: - Humidification: 138 Giles Kisby GE Y1 Respiratory o o o The alveoli of the lung must be kept moist, forotherwise respiratory exchanges cannot be carried on; the origin of this necessity is to be found in fish which left the sea to pass part of their lives on land Cold air cannot carry much moisture; therefore. if the air reaching the larynx is to be almost saturated with moisture it must first be warmed to bring its potential absolute humidity to a high level. Having warmed the inspired current, the nose can then charge it with the required amount of water Similarly, the higher the altitude the less moisture the air can hold; therefore this is a further challenge when trying to keep alveoli moist to allow gas exchange when at altitude - Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. Myoglobin is only found in the bloodstream after muscle injury. It is an abnormal finding, and can be diagnostically relevant when found in blood o Is not affected by 23DPG - Oxygen disociation haemaglobin curve to the left: o increase pH, o decrease oCO2, o decrease temperature o decrease 2,3,DPG To the right: o decrease pH o increase co2 o increase temperature o increase 2,3 DPG - - Half of airway resistance lies in the nose, pharynx and larynx 2,3 DPG lowers the affinity of o2 for haemalgobin O2 binding to Hb releases H+ due to conformational changes in the Hb - The Bohr effect is a physiological phenomenon first described in 1904 by the Danish physiologist Christian Bohr, stating that hemoglobin's oxygen binding affinity is inversely related both to acidity and to the concentration of carbon dioxide - The Haldane effect: oxygenated blood has a reduced capacity for carbon dioxide. (Conversely, Deoxygenation of the blood increases its ability to carry carbon dioxide; this property is the Haldane effect) 139 Giles Kisby GE Y1 Respiratory - Foetal Hb: Functionally, fetal hemoglobin differs most from adult hemoglobin in that it is able to bind oxygen with greater affinity (ie shift to left) than the adult form, giving the developing fetus better access to oxygen from the mother's bloodstream. - General possibilities for forms of gas in the blood: o Dissolved gas only dissolved gas molecules contribute to the partial pressure N2 is only carried in this form o Bound gas Eg O2, CO2, CO o Chemically modified gas EG CO2 HCO3- - (Bohr: left to right; Haldene: right to left) - Bohr effect: - Affinity of hemoglobin to O2 decreases when pH of blood falls / co2 inc - Facilitates release of O2 in tissues: Increased CO2 in blood --> Increased H+ production -> H+ binds to deoxyhemoglobin --> accesibility of O2 to Hemoglobin decreases --> O2 released - Haldene effect: - Binding of 02 with Hemoglobin tends to displace CO2 from blood (opposite of Bohr's effect) - Facilitates release of CO2 in lungs: HHb + O2 --> HbO2 + H+; H+ + HCO3- --> CO2 + H20 - Methods of CO2 transport in the blood: CO2 is carried in blood in three different ways. (The exact percentages vary depending whether it is arterial or venous blood): o 10% is dissolved in the plasma, 140 Giles Kisby GE Y1 Respiratory o o 5% – 10% is bound to hemoglobin as carbamino compounds. Hemoglobin, the main oxygen-carrying molecule in red blood cells, carries both oxygen and carbon dioxide. However, the CO2 bound to hemoglobin does not bind to the same site as oxygen. Instead, it combines with the Nterminal groups on the four globin chains. [carbaminoheamoglobin; nb carboxyhaemoglobin is different and is the binding of CO to HB]. However, because of allosteric effects on the hemoglobin molecule, the binding of CO2 decreases the amount of oxygen that is bound for a given partial pressure of oxygen. The decreased binding to carbon dioxide in the blood due to increased oxygen levels is known as the Haldane effect, and is important in the transport of carbon dioxide from the tissues to the lungs. A rise in the partial pressure of CO2 or a lower pH will cause offloading of oxygen from hemoglobin, which is known as the Bohr effect. Most of it (about 70% to 80%) is converted to bicarbonate ions HCO−3 by the enzyme carbonic anhydrase in the red blood cells, by the reaction CO2 + H2O → H2CO3 → H+ + HCO− In the tissues, CO2 is produced from aerobic metabolism. CO2 then diffuses across the cell membranes and across the capillary wall, into the red blood cells. The transport of CO2 across each of these membranes occurs by simple diffusion, driven by the partial pressure gradient for CO2. 2. Carbonic anhydrase is found in high concentration in red blood cells. It catalyzes the hydration of CO2 to form H2CO3. In red blood cells, the reactions are driven to the right by mass action because CO2 is being supplied from the tissue. 3. In the red blood cells, H2CO3 dissociates into H+ and HCO3-. The H+ remains in the red blood cells, where it will be buffered by deoxyhemoglobin, and the HCO3 is transported into the plasma in exchange for Cl (chloride). 4. If the H+ produced from these reactions remained free in solution, it would acidify the red blood cells and the venous blood. Therefore, H+ must be buffered so that the pH of the red blood cells (and the blood) remains within the physiologic range. The H+ is buffered in the red blood cells by deoxyhemoglobin and is carried in the venous blood in this form. Interestingly, deoxyhemoglobin is a better buffer for H+ than oxyhemoglobin: By the time blood reaches the venous end of the capillaries, hemoglobin is conveniently in its deoxygenated form (i.e., it has released its O2 to the tissues). There is a useful reciprocal relationship between the buffering of H by deoxyhemoglobin and the Bohr effect. The Bohr effect states that an increased H concentration causes a right shift of the O2hemoglobin dissociation curve, which causes haemoglobin to unload O2 more readily in the tissues; thus, the H generated from tissue CO2 causes hemoglobin to release O2 more readily to the tissues. In turn, deoxygenation of hemoglobin makes it a better buffer for H. 141 Giles Kisby GE Y1 Respiratory - 5. The HCO3 produced from these reactions is exchanged for Cl across the red blood cell membrane (to maintain charge balance), and the HCO3 is carried to the lungs in the plasma of venous blood. Cl-HCO3 exchange, or the Cl shift, is accomplished by an anion exchange protein called band three protein (so called because of its prominence in an electrophoretic profile of blood). All of the reactions previously described occur in reverse in the lungs Diffusion vs perfusion limited: o Diffusion-limited gas exchange: means that the total amount of gas transported across the alveolarcapillary barrier is limited by the diffusion process. In these cases, as long as the partial pressure gradient for the gas is maintained, diffusion will continue along the length of the capillary. Eg CO O2 during strenuous exercise / emphysema / fibrosis o Perfusion-limited gas exchange 142 Giles Kisby GE Y1 Respiratory means that the total amount of gas transported across the alveolar/capillary barrier is limited by blood flow (i.e., perfusion) through the pulmonary capillaries. In perfusion-limited exchange, the partial pressure gradient is not maintained, and in this case, the only way to increase the amount of gas transported is by increasing blood flow Eg N2O CO2 O2 under normal conditions O2 at altitude [unless compounded by exercise / emphysema / fibrosis] 143 Giles Kisby GE Y1 Respiratory - Exercise: o Adaptations during exercise: Increased alveolar ventilation Increased cardiac output Hb is near the O2 of the alveoli 3x longer than is necessary while person is at rest so the cardiac output can be increased without reduction in O2/Hb binding events Recruitment of upper lobe capillaries - High altitude; o Potential pathologies: HAPE Cerebral oedema o Adaptations at altitude: Increased ventilation Increased RBC levels Increased diffusion capacity of lungs [referring to lobe recruitment?] Increased vascularity: more capillaries Increased ability of cells to utilise O2: increased glycolysis and aerobic metabolism - Hypoventilation: o Neuromuscular disease o Obstructive/restrictive respiratory disease 144 Giles Kisby - GE Y1 Respiratory Resp failure: o Type I Hypoxaemia (low PaO2) with normal PaCO2 Occurs because CO2 has a lower solubility coefficient so provided it is not a breathing problem / hypoventilation the blood CO2 levels will be normal Eg pneumonia, anaemia, pulmonary fibrosis It is often caused by a ventilation/perfusion (V/Q) mismatch; the volume of air flowing in and out of the lungs is not matched with the flow of blood to the lungs. Eg. Pulmonary embolism o Type II: Both O2 and CO2 blood levels are affected: Emphysema: gives direct loss of SA so will affect the transfer of both gases Anything causing hypoventilation 145 Giles Kisby GE Y1 Respiratory 13/12/13: Airways I: Asthma and COPD: Philip W Ind Los (from booklet): [are combined Lec20/21 Los]: Lectures 20 and 21: Airways Disease. (Dr Philip Ind p.ind@imperial.ac.uk) At the end of these lectures you should be able to understand and describe..... t of airflow obstruction –distinction from restriction -responsivenesss utrophilia -COPD overlap Notes: - Preview: o o o o o o o o airways obstruction -distinction from restriction spirometry, peak flow (PEF), other measurements asthma -clinical importance, diagnosis atopy, asthma triggers, bronchodilator response sputum eosinophilia COPD -clinical importance, diagnosis Asthma/COPD overlap practical management; treatment Guidelines - Asthma and COPD: o Asthma is a disease of the upper airways whereas COPD is a disease of the lower airways - ASTHMA o o o o 5.2 million in Britain ↑prevalence 8-fold in 30 y 1381 deaths in 2004 including 40 children <14 y Costs £889 m per year; 12.7 million working days lost /year 146 Giles Kisby GE Y1 Respiratory o o o o o 10-fold difference in prevalence in different countries eg UK vs Indonesia/Albania ‘Western life-style’ low prevalence in rural areas (farm animals, unpasteurized milk) large family size, house-hold pets in urban areas protective ‘hygiene hypothesis’ boys > girls, women >men [more boys than girls have asthmain childhood, yet more adult women than men are afflicted with asthma] o Variability: typically young atopic individuals but also older non-atopic patients: have no such allergies and the cause of their airway inflammation is unclear [Asthma may be classified as atopic (extrinsic) or non-atopic (intrinsic) where atopy refers to a predisposition toward developing type 1 hypersensitivity reaction] In both family history and due to rhinitis [inflammation of the inside of the nose caused by an allergen] Variable ‘triggers’: URTI [upper resp tract infection]; house dust; pollen; animal fur; exercise; cold air; pollution Variable suspected causes: obesity; atopy; smoking; aspirin-induced asthma; occupational asthma eosinophilic vs non-eosinophilic variable symptoms Variable degree of airflow obstruction / severity mixed disease: asthma/COPD overlap o Asthma symptoms [variability exists] wheeze breathlessness chest tightness cough (phlegm) nocturnal and early morning symptoms o Diagnosis of asthma typical symptoms atopic ‘background’ reversible airway narrowing bronchodilator response bronchial challenge gives response (re spirometry see below) airway inflammation: ↑eosinophils in sputum (induced) o o o hyper-responsivity to various stimuli occurs characteristic airway inflammation; mast cells localise to smooth muscle corticosteroid responsive; help treat bronchial hyperresponsiveness [easily triggered bronchospasm] b2 agonists; Long acting b2 agonists: give bronchodilation [nb salbutamol] o 147 Giles Kisby GE Y1 Respiratory o Aspects contributing to the narrowing: [see image below] Smooth muscle contraction [if atopic is via allergen imm cells mediators nerves bronchoconstriction] Increased bronchial smooth muscle: enlarged smooth muscle blocks lying relatively close to the surface epithelium. Epithelial shedding / damage [even when feeling well] Inflammation and oedema Mucus hypersecretion and plasma exudation Mucus plugging can occur: Excessive mucus production admixed with inflammatory exudate forming highly tenacious plugs which block airways and are difficult to clear Gives reduced gas exchange and can cause death o Spirometry: Spirometers FEV1 measuring tubes Results typical of obstructive disease: Low FEV1; also: exercise induced fall in FEV1 Normal FVC Low FEV1 / FVC Bronchodilators improve results A bronchial challenge test is a medical test used to assist in the diagnosis of asthma.[1] The patient breathes in nebulized methacholine or histamine. Thus the test may also be called a methacholine challenge test or histamine challenge test respectively. Both drugs provoke bronchoconstriction, or narrowing of the airways. The degree of narrowing can then be quantified by spirometry. People with pre-existing airway hyperreactivity, such as asthmatics, will react to lower doses of drug. 148 Giles Kisby - GE Y1 Respiratory Airway obstruction vs lung restriction o Obstruction: ↓FEV1/VC ratio, (↓flow): asthma COPD o Restriction: ↓lung volumes (ratio may be ↑): pulm fibrosis; pleural, chest wall or resp muscle disease 149 Giles Kisby GE Y1 Respiratory 13/12/13: Airways II: Asthma and COPD: Philip W Ind Los (from booklet): [are combined Lec20/21 Los]: Lectures 20 and 21: Airways Disease. (Dr Philip Ind p.ind@imperial.ac.uk) At the end of these lectures you should be able to understand and describe..... ruction –distinction from restriction -responsivenesss ma-COPD overlap Notes: - - Preview: o o o o o o o o o o Asthma vs COPD clinically Importance of these conditions Diagnosis Asthma ‘triggers’ Bronchodilator response Airway hyper-responsivenesss Sputum eosinophilia and neutrophilia Asthma-COPD overlap Practical management Introduction to Guidelines for asthma and COPD Asthma Genetics o Complex: >100 genes implicated: IgE, BHR: bronchial hyperresponsiveness o twin studies heritability 0.36-0.77: therefore ~50% environmental o positional cloning: ADAM33, PHF11, DPP10, GRPA, SPINK5 o candidate genes: TLRs, epithelium, beta2 receptor, Th1, Th2, cytokines eg IL4, IL5, IL13, T factors, GATA 3, STAT6, FCERIB o early onset: ORMDL3, GSDML chr 17q21 signal,PDEAD chr 5q12, DENNDIB chr 1q31 o late onset: MHC genes 150 Giles Kisby - GE Y1 Respiratory Atopy: o o o o eczema, hay fever, asthma: = increased IgE skin prick test can be used to determine atopy Many asthmatic patients give a positive skin prick test to a number of allergens, all of which may contribute to their condition Serum can be analysed to determine IgE levels as mentioned atopic young people are a major subset of asthmatics - Inhaled corticosteroids: cornerstone of asthma Rx [Rx = prescription drug] o Inc lung function o o Inc bronchial responsiveness o Inc FEV1 o Dec Ag-induced bronchoconstriction o Dec asthma exacerbations o o Dec airway inflammation o ? prevent lung damage - inhaled corticosteroid and long-acting beta agonist (ICS/LABA) combination products: [convenient etc to use as combined inhaler] o ICS improve symptoms, lung function, quality of life reduce exacerbations o LABA improve FEV1, symptoms, quality of life reduce exacerbations - Leukotriene antagonists in asthma o effective in mild asthma o particularly NSAID-induced asthma o modest effects in more severe asthma o useful for nasal allergy (with asthma) o oral, well tolerated o few adverse effects o differential use in different countries - THEOPHYLLINE 151 Giles Kisby GE Y1 Respiratory o o o o o non-specific phosphodiesterase inhibitor competitive nonselective phosphodiesterase inhibitor, which raises intracellular cAMP, activates PKA, inhibits TNF-alpha and inhibits leukotriene synthesis, and reduces inflammation and innate immunity oral bronchodilator (large and small airways) anti-inflammatory beneficial effects on respiratory muscles & heart failure BUT poorly tolerated, variable pharmacokinetics and other interactions - Acute severe asthma o immediate Rx o reassurance o oxygen o high dose inh/neb salbutamol o oral prednisolone or IV hydrocortisone o high dose inh/neb ipratropium o IV Mg++ hydration, replace K+ o consider IV aminophylline (IV salbutamol) o ITU mechanical ventilation LIFE SAVING (rare) - asthma syndrome-related Asthma comorbidities: o rhinosinusitis o atopic dermatitis o asthma disease-related o small airways disease o bronchiectasis o Gastro Oesophageal Reflux o Obesity o Obstructive Sleep Apnoea - COPD: o o o Chronic obstructive pulmonary disease (COPD) is a disease state characterized by airflow limitation that is not fully reversible. The airflow limitation is is usually both progressive and associated with an abnormal inflammatory response of the lungs to noxious particles and/or gases May involve: chronic bronchitis emphysema small airways disease Facts: 152 Giles Kisby GE Y1 Respiratory o most common respiratory disease in UK 4th leading cause of death costs ~£1billion/y in UK [twice asthma] largely preventable [90% due to smoking] often undiagnosed [~50% ‘missing millions’] 10-15% die within 3/12 of hospital admission Mechanism: oxidative stress protease/anti-protease imbalance o NOXIOUS AGENTs can play role eg tobacco smoke, pollutants, occupational exposure Genetic factors can play role a1-anti-trypsin deficiency: recesssive inheritance 1% of all COPD o Alpha 1-antitrypsin (A1AT) is produced in the liver, and one of its functions is to protect the lungs from neutrophil elastase [nb neutrophil levels are high in COPD], an enzyme that can disrupt connective tissue: in individuals with the PiZZ phenotype, A1AT levels are less than 15% of normal, and patients are likely to develop panacinar emphysema at a young age Is also mentioned in the alimentary course Respiratory infection can play role Symptoms in COPD shortness of breath -progressive exercise/lifestyle limitation cough+/- phlegm difficulty producing phlegm 153 Giles Kisby GE Y1 Respiratory wheeze chest tightness nocturnal or early morning symptoms [spirometry typical of obstructive] [the emphysema can give air trapping] [stopping smoking helps; see image below] - COPD co-morbidities related to smoking o ischaemic heart disease o lung cancer o peripheral vascular disease o osteoporosis - Treatment of COPD o bronchodilators (anticholinergic or long acting β2-agonist) Tiotropium bromide: once daily inhaled cholinergic M3 receptor antagonist: ↑ lung function ↑quality of life ↑exercise endurance ↓breathlessness ↓hyperinflation ↓ exacerbations (↓hospitalisations over 12 months o inhaled steroids (for FEV1 <50%) o theophylline o STOP SMOKING o [ie Combination ICS/LABA therapy is used in COPD too (TRUE)] - Acute severe COPD [is same as for asthma] o immediate Rx 154 Giles Kisby GE Y1 Respiratory o o o o o o o o - COPD: o o o o reassurance oxygen high dose inh/neb salbutamol oral prednisolone or IV hydrocortisone high dose inh/neb ipratropium IV Mg++ hydration, replace K+ consider IV aminophylline (IV salbutamol) ITU mechanical ventilation LIFE SAVING (rare) Non-invasive ventilation used involving mask ↑ gas exchange Normalises breathing ↓ diaphragm activity Determining if there is a Aa gradient: The alveolar gas equation can be used to calculate PAO2, if the PIO2, PACO2, and respiratory quotient are known. PIO2 is calculated from the barometric pressure (corrected for water vapor pressure) and the percent O2 in inspired air (21%). PACO2 is equal to PaCO2, which is given. The respiratory quotient is assumed to be 0.8. We are told that PaO2 ; 60 mm Hg (normal; 100 mm Hg) Since the measured PaO2 (60 mm Hg) is much less than the calculated PAO2 (113 mm Hg), there must be a mismatch of ventilation and perfusion. Some blood is perfusing alveoli that are not ventilated, thereby diluting the oxygenated blood and reducing arterial PO2. The man’s PaCO2 is lower than normal because he is hyperventilating and blowing off more CO2 than his body is producing. He is hyperventilating because he is hypoxemic. His PaO2 is just low enough to stimulate peripheral chemoreceptors, which drive the medullary inspiratory center to increase the ventilation rate. His arterial pH is slightly alkaline because his hyperventilation has produced a mild respiratory alkalosis. The man’s FEV1 is reduced more than his vital capacity; thus, FEV1/FVC is decreased, which is consistent with an obstructive lung disease in which airway resistance is increased. His barrel-shaped chest is a compensatory mechanism for the increased airway resistance: High lung volumes exert positive traction on the 155 Giles Kisby GE Y1 Respiratory airways and decrease airway resistance; by breathing at a higher lung volume, he can partially offset the increased airway resistance from his disease. - Comparisons of asthma & COPD: o COPD differs from asthma in both the type of inflammation involved and in the pattern of respiratory symptoms caused o Physiologically severe asthma resembles COPD o Overlap occurs in terms of the patients: ~10-20% of patients have both conditions - Treatments common to Asthma + COPD o beta2 agonist relievers o inhaled steroids o long-acting beta2 agonists o inhaled combinations (ICS/LABA) o theophylline in severe disease o tiotropium o prednisolone for exacerbations o omalizumab (anti-IgE) - Summary o o o o o o o o o Airway obstructive diseases are common Asthma is common in young, atopic people but occurs at any age COPD -in smokers almost exclusively Asthma & COPD can co-exist inhaled therapy preferred as far as possible a multitude of inhaler devices Asthma Nurse specialists important in management esp in 10 care Self Management Plans in asthma (also in COPD) short acting beta2 agonists as ‘reliever’ bronchodilators 156 Giles Kisby GE Y1 Respiratory o o o o o o o o o o o o o inhaled steroids in asthma and more severe COPD long-acting beta2 agonists (LABAs) in asthma and COPD combination ICS/LABA inhalers in widespread use in asthma + COPD long-acting antimuscarinic (LAMA) tiotropium (Spiriva) in COPD tiotropium also in uncontrolled asthma anti-leukotrienes effective in mild asthma theophylline effective in severe asthma and COPD anti-IgE (omalizumab) in very selected, severe allergic asthma Respiratory Nurse specialists and integrated care evolving exacerbations of asthma + COPD common severe exacerbations of asthma + COPD common, life-threatening require hospital management Pulmonary Rehabilitation very cost-effective medical intervention COMMS C TUTORIAL : 14/01/14 - - The altitude, diving, sleep, coughing are prob unlikely to play big part; just odd question Says our new course convenor will stick to Los; really need to cheack that I have covered than all For him the SAQ was worth 10 marks and was split into multiple parts worth ~2 marks each; may be more than 1 now as have been doing for a few years; likely topics for that Q/Qs are: o Blood gases scenario [was his one] o Asthma and COPD o Resp failure case study If asked how would manage such a very ill patient should start with the basics before any specifics: “first take an ABCDE approach” Give O2 Lifestyle: eg stop smoking ……then any specifics Diaphragm will inc intra ab pressure during inhale Pneumothorax = air in pleural space Surface tension is the molecules of water on alveoli attracting each other Surfactant produced at 25-26 weeks ie at end of pseudoglandular stage Bronchioles can constrict: think pouseille’s law Physiological deadspace def: air inside body but unable to contribute to gas exchange 157 Giles Kisby - - - - - GE Y1 Respiratory Physiological deadspace = alveolar deadspace + anatomic deadspace Men TLC = 6L; women TLC = 4.7L Obstructive: bronchiectasis, cancer, asthma, COPD/emphysema Restrictive: mesothelioma, obesity, infection (eg pneumonia), pregnancy, kyphoscoliosis, fibrosis FVC defined as max exp after max insp FEV1/TLC = 0.8; V/Q = 0.8 Exp typically passive therefore explaining why obstructive disease is mainly a problem of expiration Curve right shift: hyperthermia, hypercapnia, acidosis o Cells working hard; require better release at tissues Curve left shift: hypothermia, hypocapnia, alkylosis o Cells don’t need more O2; dec release at tissues / uptake at lungs will occur Nb paO2 is highly dependent on the barometric pressure; normally is 760 so can achieve 100 but altitude compounded with mixing with air of high CO2 conc from prev breath means pAO2 can then start to fall below 100 meaning paO2 will too; or even lower than the PAO2 if there is a diffusion defect / altitude&exercise makes transfer perfusion limited o Note that the mixing drop means that at altitude the paO2 will never get up to barometric partial pressure (cannot equate these) ABG = arterial blood gases: o Lactate normally <2mmol/L o Can distinguish chronic meta/resp conditions by the pH: the compensation is never complete and never overshoots so if acidic then was always an acidosis o Hypoventilation: neuro, MS, drug (barbiturates, alcohol, benzodiazapenes, opiates, anaesthetics), MG, muscle wasting, restrictive lung diseases o Hyperventilation: pain, panic, anaemia o Vomiting alkylosis mech: are losing Cl from HCl so body holds onto another anion: HCO3- alkylosis o “anion gap”; will be covered in renal; cations minus anions but only Na K CL, HCO3 looked at so if gap may be ketones (ie more neg ions that the Na/K must be balancing) 1-2% of the left heart's output traverses the bronchial circulation. HPV in one area prob gives more even zonal distribution of perfusion though the paralleled underperfusion and overperfusion of diff alveoli through lung will give dec paO2 (ie the overperfusion will not yield any compensation of low O2 elsewhere as 100% saturationis max) Muscarinic antagonists could be used in asthma but are not as are never necessary; other drugs do the job Cancer general symptoms: o Night sweats o Weight loss o Fatigue TI RF said to be associated with VQ mismatch (ie underperfusion at some areas, overperfusion elsewhere) TII RF said to be associated with hypoventilation 158 Giles Kisby - - - - - GE Y1 Respiratory COPD and asma can be either class, just depends on the specific patient Failure symptoms: o Cyanosis/palor o Accessory muscle use o SOB o Nasal flaring o Pursing lips if COPD Pursing helps move the end pressure point BUT: Positive end-expiratory pressure can contribute to: Decrease in systemic venous return Pulmonary barotrauma can be caused. Pulmonary barotrauma is lung injury that results from the hyperinflation of alveoli past the rupture point. Increased intracranial pressure — In people with normal lung compliance, PEEP may increase the intracranial pressure (ICP) due to an impedance of venous return from the head.[7] Renal functions and electrolyte imbalances, due to decreased venous return metabolism of certain drugs are altered and acidbase balance is impeded SUMMARY: Lung volumes and capacities are measured with a spirometer (except for those volumes and capacities that include the residual volume). Dead space is the volume of the airways and lungs that does not participate in gas exchange. Anatomic dead space is the volume of conducting airways. Physiologic dead space includes the anatomic dead space plus those regions of the respiratory zone that do not participate in gas exchange. The alveolar ventilation equation expresses the inverse relationship between PACO2 and alveolar ventilation. The alveolar gas equation extends this relationship to predict PAO2. In quiet breathing, respiratory muscles (diaphragm) are used only for inspiration; expiration is passive. Compliance of the lungs and the chest wall is measured as the slope of the pressure-volume relationship. As a result of their elastic forces, the chest wall has a tendency to spring out, and the lungs have a tendency to collapse. At FRC, these two forces are exactly balanced, and intrapleural pressure is negative. Compliance of the lungs increases in emphysema and with aging. Compliance decreases in fibrosis and when pulmonary surfactant is absent. Surfactant, a mixture of phospholipids produced by type II alveolar cells, reduces surface tension so that the alveoli can remain inflated despite their small radii. Neonatal respiratory distress syndrome occurs when surfactant is absent. Airflow into and out of the lungs is driven by the pressure gradient between the atmosphere and the alveoli and is inversely proportional to the resistance of the airways. Stimulation of b2-adrenergic receptors dilates the airways, and stimulation of cholinergic muscarinic 159 Giles Kisby - - - - - - - - - GE Y1 Respiratory receptors constricts the airways. Diffusion of O2 and CO2 across the alveolar/pulmonary capillary barrier is governed by Fick’s law and driven by the partial pressure difference of the gas. Mixed venous blood enters the pulmonary capillaries and is “arterialized” as O2 is added to it and CO2 is removed from it. Blood leaving the pulmonary capillaries will become systemic arterial blood. Diffusion-limited gas exchange is illustrated by CO and by O2 in fibrosis or strenuous exercise. Perfusion- limited gas exchange is illustrated by N2O, CO2, and O2 under normal conditions. O2 is transported in blood in dissolved form and bound to hemoglobin. One molecule of haemoglobin can bind four molecules of O2. The sigmoidal shape of the O2-hemoglobin dissociation curve reflects increased affinity for each successive molecule of O2 that is bound. Shifts to the right of the O2-hemoglobin dissociation curve are associated with decreased affinity, increased P50, and increased unloading of O2 in the tissues. Shifts to the left are associated with increased affinity, decreased P50, and decreased unloading of O2 in the tissues. CO decreases the O2-binding capacity of hemoglobin and causes a shift to the left. CO2 is transported in blood in dissolved form, as carbaminohemoglobin, and as HCO3. HCO3 is produced in red blood cells from CO2 and H2O, catalyzed by carbonic anhydrase. HCO3 is transported in the plasma to the lungs where the reactions occur in reverse to regenerate CO2, which then is expired. Pulmonary blood flow is the cardiac output of the right heart, and it is equal to the cardiac output of the left heart. Pulmonary blood flow is regulated primarily by PAO2, with alveolar hypoxia producing vasoconstriction. Pulmonary blood flow is unevenly distributed in the lungs of a person who is standing: Blood flow is lowest at the apex of the lung and highest at the base. Ventilation is similarly distributed, although regional variations in ventilatory rates are not as great as for blood low. Thus, V/Q is highest at the apex of the lung and lowest at the base, with an average value of 0.8. Where V_/Q_ is highest, PaO2 is highest and PaCO2 is lowest. V/_Q defects impair gas exchange. If ventilation is decreased relative to perfusion, then PaO2 and PaCO2 will approach their values in mixed venous blood. If perfusion is decreased relative to ventilation, then PAO2 and PACO2 will approach their values in inspired air. Breathing is controlled by the medullary respiratory center, which receives sensory information from central chemoreceptors in the brain stem, from peripheral chemoreceptors in the carotid and aortic bodies, and from mechanoreceptors in the lungs and joints. Central chemoreceptors are sensitive primarily to changes in the pH of CSF, with decreases in pH causing hyperventilation. Peripheral chemoreceptors are sensitive primarily to O2, with hypoxemia causing hyperventilation. During exercise, the ventilation rate and cardiac output increase to match the body’s needs for O2 so that mean values for PaO2 and PaCO2 do not change. The O2-hemoglobin dissociation curve shifts to the right as a result of increased tissue PCO2, increased temperature, and decreased tissue pH. At high altitude, hypoxemia results from the decreased PO2 of inspired air. Adaptive responses to hypoxemia include hyperventilation, respiratory alkalosis, pulmonary vasoconstriction, polycythemia, increased 2,3-DPG production, and a right shift of the O2hemoglobin dissociation curve. 160 Giles Kisby - GE Y1 Respiratory Hypoxemia, or decreased PaO2, is caused by high altitude, hypoventilation, diffusion defects, _ V/_Q defects, and right-to-left shunts. Hypoxia, or decreased O2 delivery to tissues, is caused by decreased cardiac output or decreased O2 content of blood. DL / TL First CO crosses the alveolar capillary membrane (represented by ) and then CO combines with the hemoglobin in capillary red blood cells at a rate times the volume of capillary blood present ( ).[13] Since the steps are in series, the conductances add as the sum of the reciprocals: . The volume of blood in the lung capillaries, , o changes appreciably during ordinary activities such as exercise [inc thoracic pressure o o and inc Cardiac output]. Thus will appear to increase when the subject is not at rest [nb in the case of CO in addition to the inc in blood vol to enter (and therefore improved VQ matching) (ie by both diffusion and HB-binding in the case of this measure for CO) there is also an increase to Dl due to improved flow (because CO is diffusion limited – ie this component will not be reflected in a comparable DlO2 measure The lung blood volume is also reduced when blood flow is interrupted by blood clots (pulmonary emboli) or reduced by bone deformities of the thorax, for instance scoliosisand kyphosis. In disease, hemorrhage into the lung will increase its hemoglobin content, and so increase o . Finally, is increased in obesity and when the subject lies down, both of which increase the blood in the lung by compression and by gravity and thus both increase The rate of CO uptake into the blood, , o depends on the concentration of hemoglobin in that blood, abbreviated Hb in the CBC (Complete Blood Count). More hemoglobin is present in polycythemia, and o so is elevated. In anemia, the opposite is true. In environments with high levels of CO in the inhaled air (such as smoking), a fraction of the blood's hemoglobin is rendered ineffective by its tight binding to CO, and so is analogous to anemia. Varying the ambient concentration of oxygen also alters . At high altitude, inspired oxygen is low and more of the blood's hemoglobin is free to bind CO; thus is increased and appears to be increased. Conversely, supplemental oxygen increases Hb saturation, decreasing Lung diseases that reduce and and . [edit] 161 Giles Kisby GE Y1 Respiratory Diseases that alter lung tissue reduce both and to a variable extent, and so decrease . o Loss of lung parenchyma in diseases like emphysema. o Diseases that scar the lung (the interstitial lung disease), such as idiopathic pulmonary fibrosis, or sarcoidosis o Swelling of lung tissue (pulmonary edema) due to heart failure, or due to an acute inflammatory response to allergens (acute interstitial pneumonitis). o Diseases of the blood vessels in the lung, either inflammatory (pulmonary vasculitis) or hypertrophic (pulmonary hypertension). Lung conditions that increase o o .[edit] [15] Alveolar haemorrhage, polycythemia, left to right intracardiac shunts,[16] due increase in volume of blood exposed to inspired gas. Asthma due to better perfusion of apices of lung. This is caused by increase in pulmonary arterial pressure and/or due to more negative pleural pressure generated during inspiration due to bronchial narrowing.[17] 162 Giles Kisby GE Y1 Respiratory 163 Giles Kisby GE Y1 Respiratory 164 Giles Kisby GE Y1 Respiratory 165 Giles Kisby GE Y1 Respiratory BONUS: - - - - - - - - Asthma: o Running out of breath before the end of a sentence, together with a PEFR 33-50% of predicted and HR > 110/min is consistent with a severe attack of asthma. o SaO2 < 75% on air suggests a life-threatening asthma attack which should prompt immediate referral to ITU. BP 90/60 mmHg and Inaudible air entry bilaterally indicate hypotension Costochondritis = presents as severe, sharp pain radiating from anterior (typically ribs 2-5) to posterior. o A 21-year-old woman has had left-sided chest pain for a week, sharp in nature and worse on inspiration. The left medial border of her sternum is tender but her chest is otherwise clear. Myocardial infarction = pain would be on exertion not inspiration and rare in the young Pulmonary embolus = would present with tachycardia with possible hypoxia (cyanosis) and breathlessness / tachypnea, chest pain (worsened by breathing), cough / hemoptysis. The British Thoracic Society suggest that hospital admission should be seen as an ideal opportunity to review patients’ self-management skills to improve long-term asthma control: Write a plan of how and when to take the inhalers Pneumothorax = is the most likely cause of sudden pain with breathlessness in young males. The PO2 inside skeletal muscle cells during exercise is closest to 3mm Hg Clara cells have microvilli, and secrete products that are protective to the bronchial epithelium In health, physiological deadspace should equal anatomical deadspace at approx. 150ml (ie no alveolar deadspace) The most important stimulus controlling the level of resting ventilation is pH of CSF on central chemoreceptors [NOT: PCO2 on central chemoreceptors: ie PCO2 exerts its control mainly by acting via pH changes] Important subtleties of central chemosensors at medulla: o detect the changes in pH of CSF but are not sensitive to change in plasma pH because H+ are not able to diffuse across the blood–brain barrier into the CSF. Only CO2 levels affect this as it can diffuse across, reacting with H2O to form carbonic acid and thus decrease pH of CSF. o Ie central chemosensors sensitive to pCO2 only but detect pH changes only Hantaviruses are a class of viruses which famously cause Hanta Cardiopulmonary Syndrome (HCPS) and Haemorrhagic fever and Renal syndrome (HFRS). In the case of HCPS most patients present ARDS and pulmonary oedema. Influenza can cause rapidly- progressive pneumonia ± ARDS FiO2 stands for fraction (F) of inspired (i) oxygen (O2). It is expressed as a number without unit from 0.0-1.0. The normal value in the atmosphere is 0.21 (21%). It does not vary with altitude unlike partial pressure of inspired oxygen (PiO2). o At the presence of right-to-left shunting (V/Q mismatch), SaO2 might not respond to an increasing FiO2. o It may cause lung injury when >0.50 ARDS = PaO2:FiO2 ratio < 26.6. eg PaO2 = 8.0, FiO2 = 0.40 means ARDS 166 Giles Kisby - - - GE Y1 Respiratory The basal regions of the upright human lung are normally better ventilated than the upper regions because the lower regions have a small resting volume and a relatively large increase in volume. Pulmonary surfactant o Increases lung compliance o Helps to prevent transudation of fluid from the capillaries into the alveolar spaces. o Contributes to innate immunity Concerning normal expiration during resting conditions flow velocity of the gas (in cm/sec) in the large airways exceeds that in the terminal bronchioles The most important factor limiting flow rate during most (by time) of a forced expiration from total lung capacity is compression of airways 167