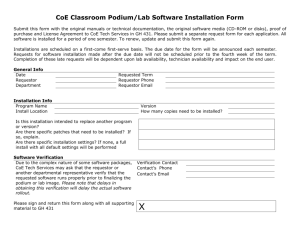
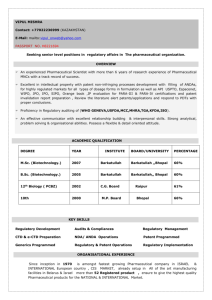
Project Request Form
advertisement

This Section for ChRi Laboratories Use Only Request Number: PRFPurchase Order Number Account No: Project Code: Date Received: Initial Reviewed by: Results Transmission: Email Fax US Mail Project Request Form Ship Samples to: ChRi Laboratories Inc 1000 Westgate Dr. Suite 241 St. Paul, MN, 55114 Send Report To: Send Invoice To: Client Project Manager: Phone Number: E-mail Address: Fax Number: Company Name: Address: City: State: Zip Code: Country: Data to be Used for Regulatory Submission? Billing Contact: E-mail Address: Quote # (if applicable): Fax Number: Company Name: Address: City: Zip Code: Request Date: Yes No Same as Send Report To Q State: Country: Due Date: Sample and Test Information Describe Sample 1. 2. 3. 4. 5. 6. ID Amount Test Code* * Test Code: See General Service Options section. Please provide additional details on work required: (Provide details for Test Code 42. Specify additional attachments): Safety and Handling Instructions (Shipping, Storage, Disposition, etc) Safety Information: Sample Storage: Sample Disposition: Container Non-Hazardous, Hazardous, Biohazard, Flammable, Potent Compound Ambient Temp., Refrigerate (2-8 oC), Freezer (-10 to -25 oC), Light Sensitive Discard, Return, Return MSDS Attached? Yes No Other Information Needed Regulatory Significance: Early Feasibility/R&D In Process Testing Method Development Required? Method Validation Required? Method Transfer Required? Use Compendia Method? Clearance (API, RM) Toxicology Studies Clinical Studies Early Pilot Studies Product Development Product Release Yes No Client Supplied Method Attached? Yes Yes No Training on Client Method Required? Yes Yes No Test Specification(s) Attached? Yes Yes No Client Supplied Reference Material? Yes Requestor Name: FRM-009, REV 1.0, 27 DEC 2010 Requestor Signature: www.chrilabs.com (612) 454 1523 Date: 2/9/2016 3:17 AM Page 1 of 2 No No No No General Service Options Test Test Code 01 02 03 04 05 06 07 08 09 10 11 12 13 14 15 16 17 18 19 20 21 Method Development Method Validation Method Transfer/Crossover Site Qualification Support Method Equivalency Studies Historic Method Validation Reference Material Qualification/Retest Assay by HPLC Content Uniformity GC Analysis Residual Solvent Analysis by GC Solvent Purity by GC Related Substance Isolation Related Substance Characterization Related Substance Identification Drug Product Characterization Karl Fischer Moisture Content Percent Solids Melting Point Residue On Ignition TLC Test Test Code USP Dissolution Studies Release Rate Studies LC/MS Analysis GC/MS Analysis Identification by FTIR Troubleshooting by FTIR Quantitative FTIR Ion Exchange Chromatography Degradation Chemistry Container Closure Studies API Solubility Studies API Intermediates Characterization Polymorph Screening & Characterization API Re-crystallization Studies API or Raw Material Clearance Stability Studies Stability Storage and Monitoring Only Photostability Studies Leachables/Extractables OOS/OOT Investigation Special Request (e.g. Early Pilot Studies) 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 Additional Stability Testing Information (Check all that apply) ICH Stability Studies ICH Photostability Studies Write Stability Protocol Shelf-Life Evaluation & Report Annual Commitment Storage Conditions: 25oC/60%RH, Issue COA after each Pull Point Write CMC Stability Section Non-formal Stability Studies 40 oC/75%RH, Other Annual Stability Report(s) Troubleshooting Stability Data Final Report Options (Check all that apply) Certificate of Analysis Brief Report (Excel Spreadsheet, Summary Tables, etc) Full Technical Report Method Validation/Verification Protocol and Report Qualification Protocol and Report Please provide additional details about report required: Test Method Instructions Method Development Report Method Transfer Protocol and Report Attach Chromatograms, Scans, Thermographs, etc. Attach Raw Data Files Please Note: A copy of the completed and signed Request Form must be submitted with samples Requestor Name: FRM-009, REV 1.0, 27 DEC 2010 Requestor Signature: www.chrilabs.com (612) 454 1523 Date: 2/9/2016 3:17 AM Page 2 of 2