Periodic Table questions
advertisement

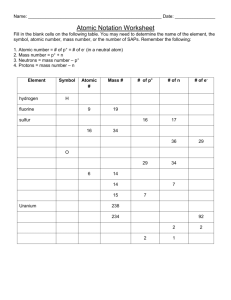
Periodic Table – Questions!! Website #1: Protons, neutrons, electrons, atomic number, element name, element symbol, mass number 1. 2. 3. 4. 5. 6. 7. How many protons are there in one atom of: (a) Carbon (b) Oxygen (c) Krypton (d) Strontium How many electrons are there in a neutral atom of: (a) Magnesium (b) Zinc (c) Sodium (d) Nitrogen What is the symbol for the following elements: (a) Silver (b) Calcium (c) Chlorine (d) Lead Name the following elements: (a) K (b) Xe (c) Fe (d) Au How many neutrons are in an atom of: (a) Phosphorus (b) W (c) Iodine (d) Beryllium What is the atomic number of: (a) Vanadium (b) Nickel (c) Hg (d) Po What is the mass number of : (a) Ge (b) Sulfur (c) Pt (d) Bismuth Website #2: groups and periods 8. Are the following groups of elements in the same group, period, or neither: a. Strontium, Zinc, Selenium b. Germanium, Carbon, Tin c. Radium, Krypton, Neon d. Francium, Sodium, Rubidium e. Antimony, Silver, Xenon Periodic Table – Questions!! Website #1: Protons, neutrons, electrons, atomic number, element name, element symbol, mass number 1. 2. 3. 4. 5. 6. 7. How many protons are there in one atom of: (a) Carbon (b) Oxygen (c) Krypton (d) Strontium How many electrons are there in a neutral atom of: (a) Magnesium (b) Zinc (c) Sodium (d) Nitrogen What is the symbol for the following elements: (a) Silver (b) Calcium (c) Chlorine (d) Lead Name the following elements: (a) K (b) Xe (c) Fe (d) Au How many neutrons are in an atom of: (a) Phosphorus (b) W (c) Iodine (d) Beryllium What is the atomic number of: (a) Vanadium (b) Nickel (c) Hg (d) Po What is the mass number of : (a) Ge (b) Sulfur (c) Pt (d) Bismuth Website #2: groups and periods 8. Are the following groups of elements in the same group, period, or neither: a. Strontium, Zinc, Selenium b. Germanium, Carbon, Tin c. Radium, Krypton, Neon d. Francium, Sodium, Rubidium e. Antimony, Silver, Xenon Website #3 and #4: group vs. period; group/family names, metals vs. nonmetals 9. To what Period number do the following elements belong: (a) Ba (b) Fluorine (c) Indium (d) Mn 10. To what Group number do the following elements belong: (a) Chromium (b) Titanium (c) Nb (d) Ar 11. To what Group/Family do these elements belong: (a) Cadmium (b) Silicon (c) He (d) Aluminum 12. What elements have properties of both metals and nonmetals? 13. The majority of the elements on the periodic table are: metals or nonmetals? 14. What alkaline earth metal has 56 protons in its atoms? 15. What transition metal has the symbol Tc? 16. Which Nobel gas has 48 neutrons? 17. Which element in Period 2 has a mass number of 19? 18. Which elements are the only liquids at room temperature? 19. Most of the ‘other nonmetals’ exists in which state of matter? 20. (a)Which Halogen reacts with Sodium to form table salt? (b) What Group/Family does Sodium belong? Website #3 and #4: group vs. period; group/family names, metals vs. nonmetals 9. To what Period number do the following elements belong: (a) Ba (b) Fluorine (c) Indium (d) Mn 10. To what Group number do the following elements belong: (a) Chromium (b) Titanium (c) Nb (d) Ar 11. To what Group/Family do these elements belong: (a) Cadmium (b) Silicon (c) He (d) Aluminum 12. What elements have properties of both metals and nonmetals? 13. The majority of the elements on the periodic table are: metals or nonmetals? 14. What alkaline earth metal has 56 protons in its atoms? 15. What transition metal has the symbol Tc? 16. Which Nobel gas has 48 neutrons? 17. Which element in Period 2 has a mass number of 19? 18. Which elements are the only liquids at room temperature? 19. Most of the ‘other nonmetals’ exists in which state of matter? 20. (a)Which Halogen reacts with Sodium to form table salt? (b) What Group/Family does Sodium belong?