NPH_4190_sm_NotesS4
advertisement

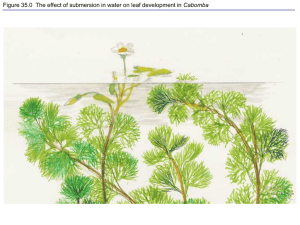
1 Supporting Information Notes S4 Molecular constraints of Pi remobilisation from senescing leaves The leaf is a sink for P, N, and other mineral nutrients during the early stages of its development. However, leaves become a nutrient source once senescence begins. P, N, K, S and several other mineral elements are mobilised from senescing leaves to new growth or developing seeds. Maximising P remobilisation from senescing leaves using selective breeding and/or biotechnological strategies will help to generate P-efficient crops that would minimise the use of unsustainable Pi fertilisers in agriculture. In maturing bean plants leaf P remobilisation accounted for over 50% of the seed P (Snapp & Lynch, 1996). P salvage from older leaves has the obvious adaptive value that it reduces the need to take up P from soil that may be poorly available or that is energetically costly to acquire (Leopold, 1961). A first step in this process is the breakdown of leaf cell components resulting in the mobilisation of nutrients. The initial target of senescence-mediated catabolism is the chloroplast (Smart, 1994; Noodén et al., 1997). It is from this organelle that much of the P and other nutrients salvaged from senescing leaf cells appear to originate (Noodén & Leopold, 1988). Small ‘senescence-associated vacuoles’ (SAVs) with intense proteolytic activity accumulate in senescing leaves of soybean, Arabidopsis and tobacco (Martínez et al., 2008). Although SAVs play a pivotal role in N-remobilisation from chloroplastic components, future research is needed to establish their role in P-remobilisation. Indeed, studies on P remobilisation from senescing leaves are relatively scarce. However, depending upon the species being investigated considerable variation appears to exist in this capability. For example, soybean only mobilises about 50% of its total P during leaf senescence (Crafts-Brandner, 1992). Similar values have been reported for a range of other crop plants. By contrast, a 78% 2 reduction in total P was reported during Arabidopsis leaf senescence (Himelblau & Amasino, 2001). There is a paucity of metabolomic data on the precise distribution of P between various inorganic and organic-P pools (e.g. Pi, nucleic acids, phospholipids, various P-esters) in senesced leaves. Defining these profiles during senescence sets the stage for experiments designed to identify senescence-associated genes (SAGs) related to leaf P remobilisation. With availability of its entire genome sequence, and a host of related genomic tools, Arabidopsis has become an effective model organism for the study of nutrient mobilisation during senescence. Transcriptomic studies using microarrays indicate that more than 800 Arabidopsis genes are distinctively up-regulated during senescence which illustrates the dramatic alteration in cellular physiology that underlies leaf senescence. 1) RNase. Although considerable amounts of Pi are present in P-lipids and P-monoesters, the nucleic acids, in particular rRNA, are an important source of C, N and especially P. The level of DNA in a senescing cell appears to remain relatively constant as senescence progresses. By contrast, rRNA levels steadily decrease during leaf senescence (Makrides & Goldthwaite, 1981), correlated with increased RNase activity (Green, 1994). Three RNase genes showing increased expression under nutritional Pi deficiency have been identified in Arabidopsis. One of these genes, RNS2, is also induced at high levels during senescence and is likely to be important for the degradation of RNA and Pi remobilisation in senescing leaves (Taylor et al., 1993; Bariola et al., 1994). The decay of rRNA in mature leaves of Arabidopsis depends on RNS2, a conserved member of the RNase T2 family (Hillwig et al., 2011). Interestingly RNS2 and two other T2-RNases, RNS1 and At1g14220, are P-starvation and senescence-induced at the transcript and/or protein level in Arabidopsis thaliana (Taylor et al., 1993; Bariola et al., 1999; Morcuende et al., 2007), thus providing a potential mechanistic link to rRNA degradation during P- 3 limitation. This is likely to be a general link in plants, as the gene encoding AhSL28, an Slike RNase from Antirrhinum hybrids that is most similar to RNS2, is also senescence- and phosphate starvation-induced (Liang et al., 2002). The functional potential of the products of RNase T2 family activity is intriguing. In yeast, RNase T2 family member Rny1p cleaves tRNA and rRNA at specific sites (Thompson & Parker, 2009). The tRNA cleavage is within the anticodon loop, producing ‘tRNA halves’, similar to those found in phloem sap (Zhang et al., 2009). These cleaved RNAs seem to act as translational inhibitors in plants (Zhang et al., 2009), providing a new signaling pathway for regulating cellular responses to stress that needs further exploration, especially in relation to the Pi deprivation response. 2) Purple acid phosphatase (PAP). PAPs undoubtedly play a pivotal role in mobilising Pi from nucleic acids and other organic-P molecules during senescence. PAPs catalyze hydrolysis of a broad and overlapping range of Pi-monoesters with an acidic pH optimum, and function in Pi production and recycling. The Arabidopsis genome encodes 29 different PAPs whose expression is influenced by various developmental and environmental factors. Recent biochemical and functional genomic studies have provided compelling evidence that the dual-targeted AtPAP26 is the predominant intracellular (vacuolar) and a major secreted PAP isozyme that functions both in vacuolar Pi recycling and extracellular Pi scavenging by Pi-starved Arabidopsis suspension cell cultures and seedlings (Hurley et al., 2010; Tran et al., 2010). The marked transcriptional induction of AtPAP17 and AtPAP26 in senescing Arabidopsis leaves (Gepstein et al., 2003) suggests that these PAP isozymes play an important role in Pi scavenging during senescence. It will be of interest to establish whether AtPAP17 and AtPAP26 activities and polypeptide levels are also up-regulated during senescence, as well as the degree to which leaf Pi remobilisation in atpap17 and atpap26 loss of function mutants has been compromised. 4 3) Pi Transport. Long-distance transport of nutrients from senescing leaves to the seeds or other parts of the plant is believed to take place via the phloem (Hill, 1980). Hence, the phloem accessibility and phloem mobility of a nutrient influences the efficiency with which it is mobilised from senescing leaves (Bukovac & Wittwer, 1957). A high-affinity phosphate transporter, PhPT1 (PhPht1;1), was cloned from senescing petunia corollas by RT-PCR (Chapin & Jones, 2009). PhPT1 expression was up-regulated during wild type corolla senescence and a much smaller increase was detected during the senescence of corollas of etr1-1 petunias which have reduced sensitivity to ethylene. PhPT1 mRNA levels showed a rapid increase in detached corollas (treated at 1 d after flower opening) following treatment with low levels of ethylene (0.1 ml l-1). Transcripts accumulated in the presence of the protein synthesis inhibitor cycloheximide, indicating that PhPT1 is a primary ethylene-response gene. PhPT1 is a putative Pi transporter that may function in Pi translocation during senescence. Similar high-affinity Pi transporters play a key role in Pi acquisition during nutritional Pi deprivation. References Bariola PA, Howard CJ, Taylor CB, Verburg MT, Jaglan VD, Green PJ. 1994. The arabidopsis ribonuclease gene RNS1 is tightly controlled in response to phosphate limitation. Plant Journal 6(5): 673-685. Bariola PA, MacIntosh GC, Green PJ. 1999. Regulation of s-like ribonuclease levels in arabidopsis. Antisense inhibition of RNS1 or RNS2 elevates anthocyanin accumulation. Plant Physiology 119(1): 331-342. Bukovac MJ, Wittwer SH. 1957. Absorption and mobility of foliar applied nutrients. Plant Physiology 32(5): 428-435. 5 Chapin LJ, Jones ML. 2009. Ethylene regulates phosphorus remobilization and expression of a phosphate transporter (phpt1) during petunia corolla senescence. Journal of Experimental Botany 60(7): 2179-2190. Crafts-Brandner SJ. 1992. Phosphorus-nutrition influence on leaf senescence in soybean. Plant Physiology 98(3): 1128-1132. Gepstein S, Sabehi G, Carp MJ, Hajouj T, Nesher MFO, Yariv I, Dor C, Bassani M. 2003. Large-scale identification of leaf senescence-associated genes. Plant Journal 36(5): 629-642. Green PJ. 1994. The ribonucleases of higher-plants. Annual Review of Plant Physiology and Plant Molecular Biology 45: 421-445. Hill J. 1980. The remobilization of nutrients from leaves. Journal of Plant Nutrition 2(4): 407-444. Hillwig MS, Contento AL, Meyer A, Ebany D, Bassham DC, MacIntosh GC. 2011. RNS2, a conserved member of the RNase t2 family, is necessary for ribosomal RNA decay in plants. Proceedings of the National Academy of Sciences of the United States of America 108(3): 1093-1098. Himelblau E, Amasino RM. 2001. Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence. Journal of Plant Physiology 158(10): 1317-1323. Hurley BA, Tran HT, Marty NJ, Park J, Snedden WA, Mullen RT, Plaxton WC. 2010. The dualtargeted purple acid phosphatase isozyme Atpap26 is essential for efficient acclimation of arabidopsis to nutritional phosphate deprivation. Plant Physiology 153(3): 1112-1122. Leopold AC. 1961. Senescence in plant development - death of plants or plant parts may be of positive ecological or physiological value. Science 134(349): 1727-&. Liang L, Lai Z, Ma W, Zhang Y, Xue Y. 2002. Ahsl28, a senescence- and phosphate starvation-induced s-like RNase gene in Antirrhinum. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression 1579: 64-71. 6 Makrides SC, Goldthwaite J. 1981. Biochemical-changes during bean leaf growth, maturity, and senescence - content of DNA, polyribosomes, ribosomal-RNA, protein, and chlorophyll. Journal of Experimental Botany 32(129): 725-735. Martínez DE, Costa ML, Gomez FM, Otegui MS, Guiamet JJ. 2008. 'Senescence-associated vacuoles' are involved in the degradation of chloroplast proteins in tobacco leaves. Plant Journal 56(2): 196-206. Morcuende R, Bari R, Gibon Y, Zheng WM, Pant BD, Blasing O, Usadel B, Czechowski T, Udvardi MK, Stitt M, Scheible WR. 2007. Genome-wide reprogramming of metabolism and regulatory networks of arabidopsis in response to phosphorus. Plant Cell and Environment 30(1): 85-112. Noodén LD, Guiamet JJ, John I. 1997. Senescence mechanisms. Physiologia Plantarum 101(4): 746753. Noodén LD, Leopold AC. 1988. Senescence and aging in plants. San Diego: Academic Press. Smart CM. 1994. Gene-expression during leaf senescence. New Phytologist 126(3): 419-448. Snapp SS, Lynch JP. 1996. Phosphorus distribution and remobilization in bean plants as influenced by phosphorus nutrition. Crop Science 36(4): 929-935. Taylor CB, Bariola PA, Delcardayre SB, Raines RT, Green PJ. 1993. RNS2 - a senescence-associated RNase of arabidopsis that diverged from the s-RNases before speciation. Proceedings of the National Academy of Sciences of the United States of America 90(11): 5118-5122. Thompson DM, Parker R. 2009. The RNase Rny1p cleaves tRNAs and promotes cell death during oxidative stress in Saccharomyces cerevisiae. Journal of Cell Biology 185(1): 43-50. Tran HT, Qian WQ, Hurley BA, She YM, Wang DW, Plaxton WC. 2010. Biochemical and molecular characterization of Atpap12 and Atpap26: The predominant purple acid phosphatase isozymes secreted by phosphate-starved Arabidopsis thaliana. Plant Cell and Environment 33(11): 1789-1803. 7 Zhang SD, Sun L, Kragler F. 2009. The phloem-delivered RNA pool contains small noncoding RNAs and interferes with translation. Plant Physiology 150(1): 378-387.