Potassium Research Paper - GHS-Advanced-Chemistry-2011
advertisement

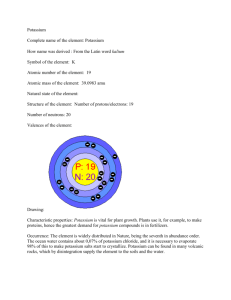
Potassium Brittany Warren December 16, 2010 In the world, pure substances are used in everyday life as we know it. Over the years scientists have called these pure substances elements. These elements have many different characteristics, looks, uses, chemical properties, masses, and symbols. One of these elements is the potassium element. Potassium gets its specific name from the combination of its historical background, specific characteristics, chemical properties, and other interesting facts. Potassium in its purest form is a silvery-white metal. It is so soft that it can be cut with a knife. On the periodic table of elements it is located right below the sodium element. Since it is located so close to sodium this means that sodium and potassium are very close chemically and are each just as abundant in nature as the other. But it is never actually found in nature by itself. Potassium forms a 1+ ion. It is in the alkali metal group, also known as Group 1 or 1A. (Baker) This group consists of lithium, sodium, rubidium, cesium and francium. “Many potassium salts are of utmost importance, including the hydroxide, nitrate, carbonate, chloride, chlorate, bromide, iodide, cyanide, sulfate, chromate, and dichromate” (Lide). Potassium originates from potash and its symbol - K - comes from the Latin word kalium (Potassium, historical information). In 1807, Sir Humphrey Davy discovered potassium. It was obtained from wood ash by boiling it and therefore reducing it to powder. Potash, a compound of potassium and a vegetable alkali, is produced when wood burns. Ashes of wood were washed with water to dissolve the potash. Its atomic number is 19 and the true atomic mass is 39.0983 amu. Like stated before, potassium is classified in the group IA, which are Alkali metals (Baker). Potassium's melting point is 63° Celsius (145° Fahrenheit) and a boiling point of 770° Celsius (1,420° F). The melting point is equal to that of a candle flame (Baker). Potassium makes up 1.5% by weight of Earth's crust and is essential to all living things. Potassium is found in plants, rocks and in the sea (Knapp). Seawater holds the largest amount of potassium. There is nearly half a kilogram of potassium oxide in each cubic meter of seawater (Knapp). Desert lakes, ancient desert rocks, and volcanic rocks are also excellent places to obtain potassium (Knapp). The atomic structure of potassium is relatively small compared to other elements. Potassium consists of nineteen protons and nineteen electrons, and twenty neutrons. The electron configuration of potassium is [Ar] 4S1. The complete writing out version can be seen in appendix B. The electron dot diagram has one dot, which represent the one valence electron. For a picture of the dot diagram, see appendix B. Potassium only has one state of oxidation, plus one. When put with water potassium it makes potassium hydroxide and hydrogen gas. This is a quick reaction and does not take very long. The equation for this is 2K + 2H2O 2KOH +H2. Another reaction is one with acids. Potassium dissolves quickly in sulfuric acid. This form K(I) ion with hydrogen gas. This equation is 2K+H2SO4 2K++SO32-+H2. (Emsley) One of potassium's compounds is sometimes used in certain types of storage batteries and in the manufacturing of liquid soap (Baker).This compound is called potassium hydroxide. Another, potassium nitrate, is used for rock salt and looks like table salt. Potassium nitrate is also used in fertilizers containing potassium (Baker). One of its biggest uses is in fireworks and gun powder (Baker). Potassium bicarbonate is found in baking soda, baking powder, antacids, soft drinks and fire extinguishers. Potassium bromide is used in photographic film and engraving (Baker). Potassium is mined in Stassfurt, Germany and in the United States (Knapp-Vol. 18). Potassium was the first metal that was isolated by electrolysis. "When using electrolysis, an electrical current creaks down the compound into its element" (Baker). Potassium in its pure form has few uses. However, compounds of potassium have many important uses; the most important is as a fertilizer. Potassium with sodium is used as a heattransfer in nuclear reactors. The alloy is liquid at high temperatures. It is also a good agent used in chemistry labs as it is a great reducing agent (Winter). Potassium hydroxide is used in the preparation for liquid detergents. Potassium is widely used in anti- freezing agents, fertilizers metal casting and welding and soldering agents. Potassium helps regulate blood pressure and heart function. It is also helpful in the contraction of nerves and muscles (Baker). Kidneys control the amount of potassium in your body (Knapp-Vol.2). In the economy today, you can buy metallic potassium is available for about $1200 per kg or $75 per g (Lide). Plant survival is based on the presence of potassium. It affects the soil health, plant growth and animal nutrition. It affects cell size, therefore causing photosynthesis and energy production as well as carbon dioxide supply. Low potassium causes restricted growth, reduced flowering, and lower quality produce. Higher levels can cause damage therefore inhibiting absorption of other minerals (Baker). Potassium has twenty four isotopes. The three main ones are potassium-39, potassium-40, and potassium-41. The isotope potassium-39 is a stable one. In the isotope potassium-40, it is unstable and is radioactive and has a half life by beta decay of 1.277 x 109 years. The isotope potassium-41 is also a stable one (“Periodic Table of Elements”). For separation scheme, begin with a water solution. Putting in sodium nitrate (NaNO3) into the water solution will start everything off. Then put sodium chlorate (Na(ClO3)3). The chlorate in that solution will then combine with silver (Ag) to make an insoluble salt. Then put in sodium hydroxide (NaOH) into the solution. The hydroxide will make the aluminum (Al) an insoluble solution with as well. Then test the potassium using a flame test. The can be seen in appendix D. The disposals of these items are safe in any way. Elements have been known around the world for many years now, one of these elements being the element potassium. Potassium is found in many different ways and in different places around the world. The element potassium has different chemical compositions than other elements on the periodic table. Potassium also has a different number of protons, neutrons, and electrons than elements around it on the periodic table. Every element is different in many different ways, these are the differences of potassium. Works Cited Baker, Lawrence W., and David E. Newton. "Potassium." P - Z. Detroit, Mich. [u.a.: U.X.L, 1999. 443-51. Print. Emsley, John. The Elements. 2nd ed. Oxford: Clarendon, 1994. Print. Knapp, Brian J. Potassium to Zirconium. Vol. 18. Danbury, CT: Grolier Educational, 2002. Print. Elements. Knapp, Brian. Sodium and Potassium. Vol. 2. Danbury, CT: Grolier Educational, 2002. Print. Elements. Lide, David R. CRC Handbook of Chemistry and Physics. Boca Raton, FL: CRC, 2008. Print. "Potassium | Historical Information." WebElements Periodic Table of the Elements. Web. 19 Dec. 2010. <http://www.webelements.com/potassium/history.html>. "Potassium | Chemical Reaction Data." WebElements Periodic Table of the Elements. Web. 19 Dec. 2010. <http://www.webelements.com/potassium/chemistry.html>. “Periodic Table of Elements.” EnvironmentalChemistry.com. J.K. Barbalace, inc., 2011. Web. 19 Dec. 2010. <http://environmentalchemistry.com/yogi/periodic/Kpg2.html#Nuclides>. Appendix A Decay Atomic Nuclide Mass Abun NN % K32 32.022 13 Syn K33 33.007 14 Syn K34 33.998 15 Syn K35 34.988 16 Syn K36 35.9813 17 Syn Spin Half Life 3/2+ 190ms 2+ DM DT ε + P Cl34 BR Energy % (MeV) 0.37 5.984 190ms ε 342ms ε + P Cl35 0.05 4.299 342ms ε + α S32 0.0034 6.166 342ms ε Ar36 12.805 ε Ar37 6.149 11.881 Ar35 K37 36.9734 18 Syn 3/2+ 1.226s K38 37.9691 19 Syn 3+ 7.636m ε Ar38 5.913 Syn 0+ 923.9ms ε Ar38 6.043 1.277E 9y β- Ca40 89.28 1.311 1.277E 9y ε Ar40 10.72 1.505 meta state 0.130MeV K39 38.9637 20 93.258 3/2+ Stable K40 39.964 21 0.012 4- K41 40.9618 22 6.73 3/2+ Stable β- Ca42 3.525 β- Ca43 1.815 β- Ca44 5.660 3/2+ 17.3m β- Ca45 4.204 Syn 2- 105s β- Ca46 7.716 28 Syn 1/2+ 17.5s β- Ca47 6.643 29 Syn 2- K42 41.9624 23 Syn 2- K43 42.9607 24 Syn 3/2+ 22.3h K44 43.9616 25 Syn 2- K45 44.9607 26 Syn K46 45.962 27 K47 46.9617 K48 47.9655 K49 48.9675 30 Syn 12.360h 22.13m 6.8s β- + N Ca47 6.8s β- 3/2+ 1.26s 1.14 12.090 Ca48 β- + N Ca48 1.26s β- 472ms β- + N Ca49 472ms β- 365ms β- + N Ca50 365ms β- 105ms β- + N Ca51 105ms β- 2.144 86 5.820 10.970 Ca49 1-, K50 49.973 31 Syn 29 7.900 214.200 Ca50 2+, K51 50.976 32 Syn 47 9.500 3/2+ K52 K53 51.983 52.987 33 34 Syn Syn 3/2+ 30ms 13.900 Ca51 >88 Ca52 β- + N Ca52 85 K54 K55 53.994 54.99 35 36 Syn 30ms β- 10ms β- + N Ca53 10ms β- Ca53 Ca54 >0 14.200 18.000 Syn (“Periodic Table of Elements”) Appendix B 𝐾∙ (Lewis dot diagram is self generated) Appendix C 1s2 2s2 2p6 3s1 3p6 4s1 Appendix D Put in NaNO3 (Sodium Nitrate) Start with water solution (H2O) Then put in Na(ClO3)3 (Sodium Chlorate) Then test the potassium with a flame test. This will react with the chlorate making silver chlorate, which is insoluble. This will react with the hydroxide making aluminum hydroxide, which is insoluble.