Endocrine_Concepts
advertisement

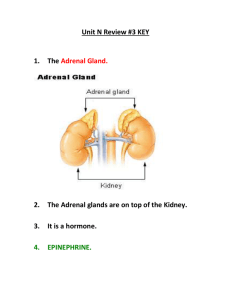
Homeostasis • Maintenance of internal environment within certain limits. • Endocrine system is one of two major systems that maintain homeostasis. • It is a “wireless” communication system that utilizes chemical messages to perform its functions. Endocrinology • Study of glands and tissues that secrete chemical messages, called hormones, which travel by way of body fluids and affect the function of target cells/tissues. Hormone Endocrine Target Paracrine Three modes of hormonal communication: long distances via the bloodstream (the classical endocrine definition), or through extracellular fluid to neighboring cell (paracrine) or on to itself (autocrine). Target Autocrine Hormones communicate by first binding to receptors. Hormones are produced in the hypothalamus, pituitary glands and in peripheral endocrine glands. • An endocrine axis involves the secretion of a specific releasing hormone &/or inhibitory hormone from the hypothalamus, (a) specific trophic hormone(s) from the pituitary and usually a third level of hormone(s) secreted from a peripheral gland. Classic Endocrine Organs Hypothalamus Releasing Hormones: GnRH, CRH TRH, GHRH Inhibitory Hormones: Somatostatin Dopamine Pituitary Gland Anterior Pituitary: ACTH, TSH FSH, LH Growth Hormone Prolactin Posterior Pituitary: Vasopressin Oxytocin Thyroid Gland Parathyroid Gland T3 , T4 Calcitonin Parathyroid Hormone Adrenal Gland Cortisol Aldosterone Androgens Estrogens Epinephrine Pancreas Insulin Glucagon Somatostatin Ovaries Estradiol Progesterone Testes Testosterone For each endocrine axis, you will learn: • Regulation of hormone synthesis biochemistry, serum protein carriers, relative t1/2, metabolism • Regulation of secretion - feedback loops • Mechanism of action of hormone Receptor/post receptor signaling • Typical symptoms of hormone excess and deficiency • Manipulation of endocrine system to enhance or suppress hormonal responses Peripheral endocrine glands include: thyroid, parathyroid, adrenal cortex, endocrine pancreas and gonads. Hormones 1. Secreted by a cell or group of cells some contained within glands. 2. Secreted into blood. Ectohormones, such as pheromones are signal molecules (alarm or attractants) secreted into the external environment. 3. Transported to a distant target tissue/cells in blood. Paracrine effects of some hormones also is recognized. 4. Exert their effects at very low concentrations; nanomolar 10-9 to picomolar 10-12 levels. 5. Act by binding to receptors. The hormone-receptor complex initiates cellular changes. 6. Action is terminated: Circulatory half-life determined by rate of hormone metabolism in liver and kidney. Hormone bound to receptors get degraded: hormone:receptor complex is often endocytosed and undergoes lysosomal degradation. Page 1 Becky Stepan Rose-Hellekant – Endo Introduction Biochemical Nature of Hormones 1) THE Peptides/protein HORMONEs: most hypothalamic & pituitary hormones coded in genome i.e., are transcribed, translated and packaged in the endocrine cell (in contrast to endocrine cells which generate steroid hormones, in that case endocrine cells take up cholesterol and convert it, via converting enzymes, to the appropriate hormone) hydrophilic so blood soluble Post-translational modification, storage, and routing of proteins assembled in ER. Protein hormones are made in cells with rough endoplasmic reticulum and are often synthesized as preprohormones in the ER are enzymatically cleaved to prohormone state and are secreted as mature hormone. Peptide hormones typically are made as inactive preprohormones Peptide hormones are made as large, inactive preprohormones that include a signal sequence, one or more copies of the hormone and additional peptide fragments that often have other biological functions. The signal sequence in (a) the preproTRH directs movement into the rough endoplasmic reticulum (RER). Preprohormones and prohormones are inactive. As pre/prohormones move through the Rough Endoplasmic Reticulum and Golgi complex they are packaged into secretory vesicles with proteolytic enzymes that lyse the protein into the various biologically active subparts and fragments shown in (a)-(c).The active peptide is secreted systemically, usually in response to a hormonal signal. Page 2 Becky Stepan Rose-Hellekant – Endo Introduction Pro-opiomelanocortin (POMC) is a precursor polypeptide with 241 amino acid residues. Background: This gene encodes a polypeptide hormone precursor that undergoes extensive, tissue-specific, posttranslational processing via cleavage by subtilisin-like enzymes known as prohormone convertases. There are eight potential cleavage sites within the polypeptide precursor and, depending on tissue type and the available convertases, processing may yield as many as ten biologically active peptides involved in diverse cellular functions. The encoded protein is synthesized mainly in corticotroph cells of the anterior pituitary where four cleavage sites are used; adrenocorticotrophin, essential for normal steroidogenesis and the maintenance of normal adrenal weight, and lipotropin beta are the major end products. ACTH is made up of 39 aa. In other tissues, including the hypothalamus, placenta, and epithelium, all cleavage sites may be used, giving rise to peptides with roles in pain and energy homeostasis, melanocyte stimulation, and immune modulation. These include several distinct melanotropins, lipotropins, and endorphins that are contained within the adrenocorticotrophin and beta-lipotropin peptides. C-peptide function is still being studied. It is often used clinically as a measure of Insulin production as 1 molecule of c-peptide = 1 molecule of insulin. Protein Hormones bind to Membrane Receptors Protein hormones are hydrophilic so dissolve nicely in water however cannot cross cell membranes. Most protein hormones bind to cell membrane receptors which traverse the lipid bilayer. Receptors can act as channels, are kinases so transfer energy via ~P, &/or G protein coupled. Responses can be both rapid (release of secretory product such as another hormone) and slow (induction of transcription after a cascade of intracytoplasmic events leading to changes in nucleus). blood Plasma membrane cytoplasm Biochemical Nature of Hormones 2) Steroid HORMONEs • Produced in peripheral endocrine organs (not in hypothalamus or pituitary gland). • Constructed from dietary or liver synthesized cholesterol • Converted in endocrine gland by enzymes in mitochondrial membrane & smooth ER • Are hydrophobic- traverse cell membranes but need carrier proteins to circulate Page 3 Becky Stepan Rose-Hellekant – Endo Introduction Steroid hormones readily traverse cell membranes (when free, i.e.not bound to carrier proteins) Carrier protein bound-steroid hormone Free hormone + carrier protein Steroid hormones are synthesized from plasma cholesterol. Steroid hormones are made in the adrenal cortex, gonads and placenta. Steroidogenic cells have large amounts of smooth ER (endoplasmic reticulum) which is where steroidogenesis occurs, as well as enzymes in the mitochondrial membranes. Steroids are lipophilic so traverse lipid membranes easily. This means they are not stored in vesicles as peptide hormones are. They generally are synthesized on demand and hormone leaves cells by simple diffusion down a concentration gradient. Because they a lipophilic, most is bound bound to carrier proteins I- this makes it miscible in the circulation. There is an equilibrium between bound and free steroid hormone in circulation. Some carrier proteins are specific to the hormone, binding hormones at 10-10 to 10-8, , however, the concentration of specific binding protein is generally low. Other proteins, such as albumin, also bind hormones but binding requires high concentrations of hormone (at 10-6 to 10-4). Binding is nonspecific in this case.. There are several implications: (1)methods of measurements of circulating hormones may measure bound and/or free hormone, (2) reductions in specific carrier protein and albumin levels (liver disease) alter this reservoir of bound hormone. (3) Steroid hormones that are bound to carrier proteins are protected from degradation. Biochemical Nature of Hormones 3) HORMONES constructed from the amino acid, tyrosine mostly synthesized in cytoplasm Can be either Hydrophilic (e.g., catecholamines) or similar to steroid hormones, hydrophobic (e.g., thyroid hormones) Mode of Hormone Transport in the Circulation • Hydrophilic hormones circulate “free” in the plasma. Can also have specific and nonspecific (albumin) binding proteins. • Hydrophobic hormones are generally bound to a specific plasma binding/carrier protein. Albumin also can bind these hormones. • There is a small amount of “free” unbound hormone and this is the active form of the circulating hormone. • Carrier/binding proteins protect hormones from degradation by liver and act as a reservoir. Page 4 Becky Stepan Rose-Hellekant – Endo Introduction Nuclear Receptor Signaling Paradigm E2 SHBG E2 2. ligand-independent GF GF R HSP R HSP R HSP R SHBG E2 E2 R E2 HSP R R E2 E2 1. classical E2 4. nongenomic SHBG R R E2 E2 R HSP E2 E2 R P P 3. ERE-independent R mRNA E2 E2 Polysomes E2 Tissue Responses Protein E2= estrogen (specifically estradiol 17beta); E circulates in the blood bound to SHBG; Free hormone binds to receptor in cytoplasm. Receptors are stabilized in cytoplasm by binding to heat shock protein (HSP). Ligand: receptor moves into the nucleus, dimerizes and binds to ERE domain which some Eresponsive genes contain (type 1: classical ERE binding. E:receptor dimers can also bind other transcription cofactors (jun/fos) which bind AP-1 sites on another type of estrogen responsive genes (type 3; EREindependent signaling). SHBG: Steroid hormone binding globulin; GF: Growth Factor HSP: Heat shock protein; E2=Estrogen; R=receptor; Under conditions when growth factor stimulation occurs, ligand-independent ER (estrogen receptor) binding to ERE sites can occur (type 2 signaling). Type 4 signaling is termed nongenomic because the ligand receptor binding occurs at the cell membrane which initiates signal cascades in the cytoplasm similar to protein hormone: receptor responses. This paradigm described for estrogen and estrogen receptor is similar for the other steroid hormones. Short = Minutes Long = Minutes - Hours Page 5 Becky Stepan Rose-Hellekant – Endo Introduction Mode of Hormone Transport in the Circulation • Hydrophilic hormones circulate “free” in the plasma. Can also • ave specific and nonspecific (albumin) binding proteins. • Hydrophobic hormones are generally bound to a specific plasma binding/carrier protein. Albumin also can bind these hormones. • There is a small amount of “free” unbound hormone and this is the active form of the circulating hormone. • Carrier/binding proteins protect hormones from degradation by liver and act as a reservoir. What is the clinical relevance of binding proteins? • bound, free or total hormone values may be presented in assays • diseases may decrease binding proteins (e.g. liver) **Peptide hormone concentration is between 10-12 mol/L to 10 -10 mol/L. Steroid and thyroid hormone concentrations are 10-9 and 10-6 mol/L respectively. The endocrine system includes the hypothalamus and pituitary glands. The pituitary gland is made of two embryologically separate structures. Neurons producing hormones have cell bodies in the hypothalamus and project tand secrete their hormones into either the hypothalamichypophyseal portal system or into the vessels bathing the posterior pituitary gland. Capillaries carrying the newly secreted hypothalamic hormones from the hypothalamic-hypophyseal portal system bathe the anterior pituitary endocrine cells. Hormones secreted into the blood supply of the posterior pituitary are immediately available in the general circulation. Most of the hormones we will be considering involve the anterior pituitary gland. Page 6 Becky Stepan Rose-Hellekant – Endo Introduction Posterior Pituitary Posterior pituitary hormones are vasopressin and oxytocin. An endocrine axis involves negative feedback so that when there is an increase of hormone produced in the peripheral endocrine gland it feeds back negatively to the hypothalamus and pituitary, reducing the release of their respective hormones (Long loop feedback). In most cases when the pituitary releases its hormone, it feeds back negatively to the hypothalamus (short loop feedback). Hormone secretion/circulating levels fluctuate during the day. Hypothalamic & pituitary hormones are released in pulses…… which is due to fluctuations of peripheral indicators (e.g. glucose) in the case of insulin or due to a hypothalamic pulse generator in the case of GnRH release. This is also true for hypothalamic and pituitary hormones. Circadian secretion of hormones also occurs with light and sleep as major environmental clues. Permissive Action So hormone deficiencies or excesses may impact the function of another hormone. Some hormones promote the action of other hormones. Page 7 Becky Stepan Rose-Hellekant – Endo Introduction Endocrine imbalances Here is an example of endocrine imbalance which occurs with the administration of exogenous hormone, in this case cortisol. High levels of circulating cortisol reduces the levels of hypothalamic releasing hormone (corticotrophic releasing hormone) and anterior pituitary hormone (adrenocorticotrophic hormone) due to long loop negative feedback. Cortisol is typically produced by the adrenal cortex. Target cells/tissues respond to cortisol (whether it is endogenous or exogenously provided). Usually excess cortisol is provided exogenously due to need to reduce immune reaction Once the reaction subsides, exogenous cortisol is withdrawn. Slow withdrawal from exogenous cortisol is necessary to allow a smooth transition back to endogenous regulation of cortisol. Too rapid of exogenous cortisol withdrawal would lead to cortisol crash because the hypothalamo- pituitary-adrenal axis is temporarily shut down. The adrenal glands are incapable of producing cortisol without a transition period to normal production levels. FYI: Look to specific lecture on adrenal gland physiology. If the endocrine imbalance, in this case hypersecretion, is due to problems at the level of the peripheral endocrine gland, then it is called “primary hypersecretion” If the problem is at the level of the hypothalamus it is known as secondary. Some references suggest that problems at the level of the pituitary is referred to as “tertiary” but others lump this into “secondary hypersecretion” along with the hypothalamus. One can distinguish the problem endocrine gland by measuring hormone levels in the peripheral blood. * Too little glucocorticoid causes symptoms of adrenal insufficiency, such as anorexia, nausea, vomiting, abdominal pain, asthenia, poor weight gain, and weight loss. * Too much glucocorticoid causes excessive weight gain, cushingoid features, hypertension, hyperglycemia, cataracts, and growth failure. * In children, growth failure is a sensitive indicator of exposure to excessive glucocorticoids. Expected circulating levels of hormone: Page 8 Becky Stepan Rose-Hellekant – Endo Introduction