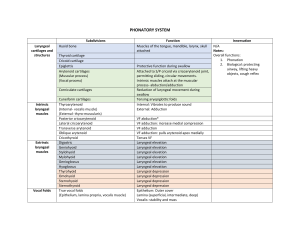
call for recruits to a clinical trial
advertisement

For the attention of laryngologists: call for recruits to a clinical trial RegenVOX Trial: A stage 2a clinical trial of stem cell based tissue engineered partial laryngeal replacement grafts Chief Investigator: Professor Martin Birchall, UCL Ear Institute We are now recruiting to the RegenVOX trial. This is a groundbreaking MRC funded clinical trial sponsored by University College London. We will use autologous stem cell based, tissue engineered partial (non-moving, but anatomically matched) laryngeal replacement grafts in 10 adult patients with advanced laryngeal and upper tracheal disorders. Following successful trialling in pigs, we have refined technology to produce and implant a partial laryngeal or upper tracheal replacement. This is composed of a human donor decellularised scaffold (obtained through UK Blood and Transplant) re-seeded with autologous (patient’s own) stem cells. We have previously achieved proof-of-principle through compassionate use tracheal implants in three patients using similar technology. The intention is to allow these rare but functionally disabled patients to lead lives with a prosthesisand tracheostomy-free airway, improve voice and swallowing function and reduce the need for repeat admissions and operations. This would benefit patients and those who care for them and reduce patient, society and healthcare costs. If successful in these patients with benign pathology, this technology could significantly extend therapeutic options for head and neck cancer patients as well, reduce rates of laryngectomies and the threshold for selecting surgery over chemoradiotherapy, thereby reducing morbidity. Inclusion criteria: Patients aged 18-70 years with Myer-Cotton Grade 3 or 4 laryngotracheal stenosis due to traumatic, iatrogenic, inflammatory or idiopathic causes who have exhausted conventional therapies. We include those with low grade malignant or benign tumours of the larynx who might be heading for laryngectomy in time, and those who have had partial laryngeal resections for laryngeal cancer and are recurrence free at a minimum of 2 years. Exclusion criteria: Pregnancy Those unable to provide informed consent Co-morbid moderate or severe chronic obstructive pulmonary disease (according to NICE criteria in CG101) Patients with active / uncontrolled chronic inflammatory conditions such as granulomatosis with polyangitis (Wegener’s) and sarcoidosis Any current or previous cancer within 5 years (except non-melanoma skin cancer, adequately treated carcinoma in situ of the uterine cervix and laryngeal malignancy treated locally without distant metastases) Life expectancy less than 5 years If you have patients who fulfil these criteria and may be interested in participating in our trial, or if you require further information about the trial then please contact the trial team via: Sophie Wilson RegenVOX Clinical Research Nurse University College London Email: sophie.wilson@ucl.ac.uk Martin Birchall Professor of Laryngology Royal National Throat Nose & Ear Hospital m.birchall@ucl.ac.uk