WE, Aerobic Glycolysis
advertisement

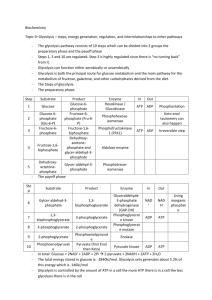
WE Presentation #2 Content to powerpoint posted Aerobic Glycolysis, lactate production and hypoxia. The conversion of glucose to lactic acid, which can occur in hypoxic normal cells, persistent cancer cells despite the presence of oxygen that would normally inhibit glycolysis through a process termed the Pasteur Effect. We now know that sustained aerobic metabolism through glycolysis diminished Pasteur Effect and in certain cancer cells it is linked to the activation of oncogenes or loss of tumor suppressors. The Warburg Effect came after Pasteur’s findings and was with cancer cell, where Pasteur was looking at yeast and the relationship of anaerobic and aerobic production of ATP. As neoplastic cells accumulate in threedimensional multicellular mass, local low nutrient and oxygen levels triggers the growth of new blood vessels in the neoplasm. The imperfect neo-vasculature in the tumor bed is poorly formed and inefficient and hence poses nutrient and hypoxic stress. Glucose is transported into cells by facilitated transporters and then trapped intracellular by glucose phosphorylation. The hexose phosphate is further phosphorylated and split into 2, 3 Carbon molecules (GAP & DHAP) that are converted to glycerol for lipid synthesis or sequentially transformed to pyruvate. Pyruvate is transported into the mitochondria and is converted to acetyl-CoA were it condenses with OAA to form citrate in the TCA cycle. Formation of citrate from acetyl CoA and OAA permits a new round of TCA cycling, generating high-energy electrons, CO2, and carbon skeletons that can be used for biosynthesis. Citrated self could be extruded into the cytosol and then converted to acetyl-CoA a by ATP-dependent citrate lyase for fatty acid synthesis to produce phospholipids and generate new biomembranes. Glucose through the pentose phosphate pathway generates ribose for nucleic acid synthesis and NADPH for reductive biosynthesis, and to reduce reactive oxygen species (ROS). Glutamine, which circulates with the highest concentration amongst amino acids, serves as a major bioenergetic substrate and nitrogen donor for proliferating cells. Glucose and glutamine are required for hexose amine biosynthesis. Glutamine enters the TCA cycle via the deamination to glutamate and then to αKG a key TCA cycle intermediate. Under hypoxia, the hypoxic inducible factor (HIF – 1) activates pyruvate dehydrogenase kinase that inhibits pyruvate dehydrogenase and the conversion of pyruvate to acetyl-CoA, thereby shunting pyruvate to lactate through homolactic fermentation. Allosteric regulations of glycolysis (Figure 4 of WE video presentation) confer metabolic plasticity with respect to local pO2. Enzymes are represented in italicized blue font and their substrates in bold black. Because the glycolytic flux is nominally faster than OXPHOS, the Pasteur Effect has been evolutionary selected to couple both metabolic rates. The energy metabolites glucose-6-P, ATP, and citrate restrain the glycolytic flux through allosteric inhibition of key 1 glycolytic enzymes, as represented by the red arrows. Inhibition is at its climax when oxygen is not a limiting substrate for OXPHOS, thus allowing the full oxidation of glucose. When oxygen levels are limited or when the pO2 fluctuates, full glucose oxidation, and consequently the levels of ATP and citrate produced oxidatively are decreased. The Pasteur Effect is reset to less pronounced inhibition, thus allowing accelerated glycolysis to compensate for defective ATP production. An extreme situation characterized by full inhibition of the Pasteur Effect is met under severe hypoxia. The energetic crisis is associated with an increase in the cellular levels of fructose-1,6-BP, ADP, AMP, and inorganic phosphate (Pi). These molecules exert a series of allosteric stimulations (represented by the green arrows) that accelerate the glycolytic flux. Glycolysis thus becomes the main source of cellular ATP production, a rescue situation allowing short-term cell survival until the pO2 is restored. Other abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; P, phosphate; PPP, pentose phosphate pathway; TCA, tricarboxylic acid (cycle). http://www.frontiersin.org/files/Articles/13090/fphar-02-00049HTML/image_m/fphar-02-00049-g001.jpg The best-characterized regulatory mechanism is that modulating HIF-1α’stability. Under well-oxygenated normoxic conditions, prolyl hydroxylation (by prolyl hydroxylases [PHDs]) and subsequent ubiquitination (by von-Hippel Lindau (VHL)-containing E3 ubiquitin-protein ligase) of the oxygen-dependent degradation (ODD) domain of HIF-1α leads to rapid degradation of HIF-1α with a half life of 5-8 min. Consequently, HIF-1 is inactive under normoxic conditions On the other hand, under oxygen-deprived hypoxic conditions, HIF-1α becomes stable because oxygen-depletion directly decreases the PHDs’ activity. Then, HIF-1α interacts with HIF-1β, forms a heterodimer, HIF-1, binds to its cognate DNA sequence, the hypoxic-responsive element (HRE), and finally induces the expression of various genes related to angiogenesis, metastasis and glycolysis .In addition to the regulation of HIF-1α’s stability, another post-translational modification of HIF-1α is known to function in the regulation of the transactivational activity of HIF-1. Under normoxic conditions, factor inhibiting HIF-1 (FIH-1, Hypoxia-inducible factor 1-alpha inhibitor is a protein that in humans is encoded by the HIF1AN gene), becomes active and hydroxylates an asparagine residue (N803) of HIF-1α. The asparaginyl hydroxylation blocks the interaction of HIF-1α with the transcriptional co-factor p300 and CBP, resulting in the suppression of HIF-1’s trans-activational activity. Because oxygen is a substrate of FIH-1 as well as PHDs, HIF-1’s trans-activational activity is restored under oxygen-deprived hypoxic conditions. http://www.intechopen.com/source/html/17924/media/image3.png HIF-1 controls metabolic and pH-regulating pathways. Cells respond to hypoxia by HIF-1-mediated upregulation of glucose transporters (Glut-1 and Glut-3) and enzymes of glycolysis, hexokinase (HK), phoshofructokinase-1 (PFK-1) and pyruvate kinase (PK). Conversion of pyruvate to lactic acid is facilitated by the induction of lactate dehydrogenase (LDH). HIF-1 also induces pyruvate 2 dehydrogenase kinase-1 (PDK-1), which inhibits the conversion of pyruvate into acetyl-CoA by pyruvate dehydrogenase (PDH), thus preventing entry of pyruvate into the TCA cycle. Subunit composition of cytochrome coxidase (COX4) is influenced by HIF-1 in hypoxia: COX4-2 is induced and COX4-1 is reciprocally reduced by induction of the protease LON that degrades COX4-1. Switching the COX subunits ensures optimal efficiency of mitochondrial respiration in hypoxia. Furthermore, pH homeostasis is maintained by induction of carbonic anhydrase IX (CAIX) and the monocarboxylate transporter MCT 4 and the Na+|[sol]|H+ exchanger NHE1. http://www.nature.com/cdd/journal/v15/n4/images/cdd200812f2.jpg 3