5.11.15.A.Tarvin.LessonPlans.Unit10.Week18
advertisement

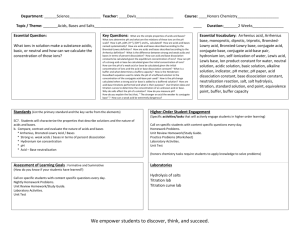
Monday Content Standard: Explain the process of dissolving in terms of solute/solvent interactions: Observe factors that affect the rate at which a solute dissolves in a specific solvent Express concentrations as molarities Compare, contrast, and evaluate the nature of acids and bases o Arrhenius and Bronsted-Lowry Acid/Bases o Strong vs. weak acids/bases in terms of percent dissociation o pH o Acid-Base neutralization Essential Question: How are neutralization reactions carried out in the lab? What calculations are necessary to titrate correctly? Musical Theme: Motown Monday Learning Activities: [30 minutes] Whiteboard Review of pH, pOH, and kw Summary: Teacher will facilitate a warm-up and review of last week’s calculations. Students will have the opportunity to ask questions about their tiered assignments. [30 minutes] pH, pOH, kW Quiz Summary: Students will demonstrate mastery of calculation skills on a 15-question quiz. [30 minutes] Titrations Summary: Students will view their last chem-to-go videos. They will learn how pH and molarity are used in titration reactions Differentiation Plan: Flexible grouping: Students are purposefully grouped homogeneously. Assessment Plan: Formal assessment of understanding: Teacher will grade, mark, and return the students’ quizzes. Homework: Prepare for tomorrow’s virtual titration lab. Tuesday Content Standard: Explain the process of dissolving in terms of solute/solvent interactions: Observe factors that affect the rate at which a solute dissolves in a specific solvent Express concentrations as molarities Compare, contrast, and evaluate the nature of acids and bases o Arrhenius and Bronsted-Lowry Acid/Bases o Strong vs. weak acids/bases in terms of percent dissociation o pH o Acid-Base neutralization Essential Question: How are neutralization reactions carried out in the lab? What calculations are necessary to titrate correctly? Musical Theme: Twangy Tuesday Learning Activities: [60 minutes] Virtual Titrations Lab Summary: Students will carry out a virtual titration. The lab activity requires them to manipulate the addition of a base to a known acid. Students encounter real-life problems that might require them to start over or revise their calculations. Students will carry out three different trials. [30 minutes] Titrations Practice Summary: Students will solve titration calculations to prepare for tomorrow’s titration quiz. Differentiation Plan: Appropriate challenge: Every student will encounter a different set of variables in the lab. Assessment Plan: Formal assessment of understanding: Teacher will grade, mark, and return the students’ labs. Homework: Prepare for Thursday’s lab practical and cumulative quiz. Wednesday Content Standard: Explain the process of dissolving in terms of solute/solvent interactions: Observe factors that affect the rate at which a solute dissolves in a specific solvent Express concentrations as molarities Compare, contrast, and evaluate the nature of acids and bases o Arrhenius and Bronsted-Lowry Acid/Bases o Strong vs. weak acids/bases in terms of percent dissociation o pH o Acid-Base neutralization Essential Question: How are neutralization reactions carried out in the lab? What calculations are necessary to titrate correctly? Musical Theme: The Middle Learning Activities: [30 minutes] Titrations Quiz Summary: Students will demonstrate current understanding by solving a few titration questions on a quiz. [30 minutes] Acid/Base Review Summary: Students will move around the room and answer posted questions to review for tomorrow's lab practical and cumulative quiz. Teacher will facilitate a review of theory/definition quiz and pH quiz. [30 minutes] Final Exam Review Summary: Teacher will distribute and explain the exam review materials. Students will begin solving the first 3 units’ questions. Differentiation Plan: Flexible grouping: Students will review with a partner of similar skill level to himself/herself. Assessment Plan: Formal assessment of understanding: Teacher will grade, mark, and return the students’ labs. Homework: Prepare for tomorrow’s lab practical and cumulative quiz. Thursday Content Standard: Explain the process of dissolving in terms of solute/solvent interactions: Observe factors that affect the rate at which a solute dissolves in a specific solvent Express concentrations as molarities Compare, contrast, and evaluate the nature of acids and bases o Arrhenius and Bronsted-Lowry Acid/Bases o Strong vs. weak acids/bases in terms of percent dissociation o pH o Acid-Base neutralization Essential Question: Have I mastered the acid/base chemistry standards? Musical Theme: Throwback Thursday Learning Activities: [45 minutes] Acid/Base Lab Practical [45 minutes] Unit Nine Cumulative Quiz Differentiation Plan: All students will perform the same assignments today. Assessment Plan: Formal assessment of understanding: Teacher will grade, mark, and return the students’ lab practicals. Collaborative, consistent assessments: Lab practical and cumulative quiz were developed collaboratively with other HHS chemistry teachers. Homework: Prepare for tomorrow’s unit test. Friday Content Standard: Explain the process of dissolving in terms of solute/solvent interactions: Observe factors that affect the rate at which a solute dissolves in a specific solvent Express concentrations as molarities Compare, contrast, and evaluate the nature of acids and bases o Arrhenius and Bronsted-Lowry Acid/Bases o Strong vs. weak acids/bases in terms of percent dissociation o pH o Acid-Base neutralization Essential Question: Have I mastered the acid/base chemistry standards? Musical Theme: Relaxation Station Learning Activities: [90 minute] Unit Ten Acid/Base Test Differentiation Plan: All students will perform the same assignments today. Assessment Plan: Formal assessment of understanding: Teacher will grade, mark, and return the students’ tests. Collaborative, consistent assessments: Unit tests were developed collaboratively with other HHS chemistry teachers. Homework: Prepare for the final exam.