Name____________________________ Block______
advertisement

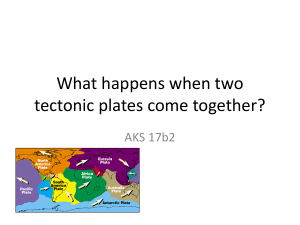
Name____________________________ Block______ Homeroom_____ SCA Review The following review is a brief outline of the major objectives learned this year. It provides some tips to avoid common mistakes as well as recommended test strategies. Week 1- Feb 23-27 Know that an element is a pure substance. Pure substances- no other elements at all (Ex: Hg, C, O2) There may be one atom (Ex: Hg) There could also be more than one atom (Ex: H2) Recognize that a limited number of elements make up most of Earth. 6 most common elements- Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus, Sulfur Nitrogen is most common element of all on planet Earth Nitrogen in most common in the atmosphere Oxygen is most common element in the crust, oceans, and living matter Differentiate between elements and compounds. Chemical symbols- find on periodic table, can be one or two letters, only first letter a capital letter Compounds are 2 or more elements (Ex: H2O, NaCl) Mixtures are more than one molecule that have not been chemically combined Test Strategy- underline all capital letters in a chemical formula. If there is only 1 capital letter it is pure, if there are 2 or more capital letters it is a compound. Week 2- March 2-6 Compare metals, nonmetals, and metalloids. Metal, Nonmetals, Metalloids- know if each is a good conductor or not Calculate the density of unknown substance. Density=m/v Density is how compact the molecules are, if you cut an object into pieces those pieces have the same density at the whole Density misconceptions- the bigger the object the more dense(this is wrong!) Density changes with temperature- cold causes molecules to come together and be more dense, warming causes them to spread apart be less dense Identify the formation of a new substance as a sign of a chemical change. 4 signs of chemical change = gas, heat, color, precipitate Name____________________________ Block______ Homeroom_____ SCA Review Week 3- March 16-20 Compare and contrast potential and kinetic Pendulum- at the top the object has the most potential energy, the bottom has the most kinetic energy until it changes direction Kinetic energy is affected by mass and speed Potential energy is affected by mass and position Identify and describe changes in position, direction and speed when acted upon by unbalanced forces. Opposing forces that are balanced = 0N net force Acceleration is speeding up, slowing down, or changing direction Investigate how inclined planes and pulleys can be used to change the amount of force to move an object. Simple machines keep the work the same Simple machines require less force to get the same amount of work done Week 4- March 23-27 Calculate average speed. Speed=d/t On a graph, look at the last point on the graph and use the distance and time for that point ONLY Measure and graph change in motion. Change of motion can be shown on a graph o Straight line = constant motion o Curve up/down = acceleration o Flat line = no motion (stopped) Distance Graph misconception- the line shows the path the object took- No! Time This arch does not show an object going up and then down, it shows accelerating, stopping and then returning in the direction it came from. Name____________________________ Block______ Homeroom_____ SCA Review Week 5- 30-April 3 Investigate methods of thermal energy transfer. Conduction- heat objects by direct contact, convection- heat rises in air or liquid, radiation- heat travels through space and only warms the objects not the space Heat always moves from warm to cool until temperature is balanced Verify that thermal energy moves from warm to cool. Example: when you sit on a cold chair it will gradually warm up. The thermal energy from you moves to the colder chair. Demonstrate energy transformations. Types of energy: thermal, radiant, electrical, chemical, mechanical, nuclear Power plants transform energy in several steps o Example: Coal Power Plant Chemical energy in coal is burned and turns into thermal energy. Thermal energy is used to boil water and create steam. Steam moves and so the thermal energy had changed into mechanical energy. Moving steam spins the blades of a turbine which are connected to a generator. A generator changes the mechanical energy into electrical energy. Conversion examples o Batteries to flashlight (chemical to electrical to radiant) o Sunlight to photosynthesis (radiant to chemical) o Food to riding a bike (chemical to mechanical) o Wind to electricity (mechanical to electrical) Week 6- April 6-10 Research and debate the advantages and disadvantages. Fossil fuels currently the most used energy source in the U.S. Renewable: biomass, trees, plants, wind, water, hydroelectric, geothermal Nonrenewable: coal, oil, natural gas Design a logical plan to manage energy sources. Ways to conserve electricity: turn off lights, unplug appliances, switch to lower watt light bulbs, use less AC in the summer and less heat in the winter Build a model to illustrate the layers of Earth. Litho = rock The tectonic plates make up the crust and sit on top of the asthenosphere (move) Layers of Earth: inner core, outer core, mantle, asthenosphere, lithosphere, crust Name____________________________ Block______ Homeroom_____ SCA Review Week 7- Apr 13-17 Identify the major plates. 6 major plates: African, North American, South American, Eurasian, IndoAustralian, Pacific Describe hose plate tectonics causes major geological events Convergent Boundary- comes together, Divergent Boundary- move apart, Transform Boundary- slide past The tectonic plates move by sitting on convection currents that are in the asthenosphere Geological events are created by plate movement o Ocean basins- divergent (cont./cont.) o Earthquakes- transform o Volcanic eruptions- convergent (subduction), transform o Mountain building- convergent (cont./cont.) o Ocean Ridge- divergent (oceanic/oceanic) o Seafloor spreading- divergent (oceanic/oceanic) o Island formation- convergent (subduction) (oceanic/oceanic or oceanic/cont.) o Trenches- divergent o Faults- transform o Rift- divergent (oceanic/oceanic) o Rift Valley- divergent (cont./cont.) Name____________________________ Block______ Homeroom_____ SCA Review Week 8- Apr 20-24 Test the physical properties of minerals. Hardness- use scratch test with Moh’s hardness scale Streak-color of mineral Luster-shine Magnetism- is it magnetic Cleavage- describes what pattern the mineral breaks in Texture-feel Color- color when observing Classify rocks based on the process of their formation. Rock Types Processes that Form Rocks Metamorphic Melting Sedimentary Cooling igneous Layering Compaction Cementation Heating pressure Metamorphic- heat and pressure Sedimentary- compaction, cementation, and layering Igneous- melting and cooling Week 9- Apr 27- May 1 Describe the physical properties, locations, and movements of planets, moons, starts, and space objects. The Sun is a medium sizes star in the center of solar system 8 major planets- know order, characteristics, composition, moons Rotation is spinning (day), revolution is orbiting (year) Asteroid- most found in belt between Mars and Jupiter, they are made of rock and orbit the Sun Comets- made of ice, elliptical orbit, tail points away from Sun Meteoroid- rocks traveling in space, burns up in atmosphere-called a meteor, lands on Earth-called a meteorite Larger planets have more gravity because they have more mass Understand that gravity is the force that controls all movement in the solar system. Weight changes when gravity changes, mass of an object stays the same Gravity pulls planets toward the Sun, Inertia keeps planets moving in their orbit (planets would shoot out in a strait line if gravity disappeared) Light travels in space since it is energy, sound does not travel in space since it needs air molecules to vibrate and there is no air in space