Absorption spectra of TCHQ in various solvents TCHQ in Ether
advertisement

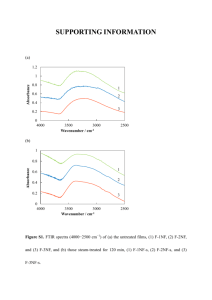
Absorption and fluorescence emission attributes of a fluorescent dye: 2,3,5,6-tetracyano-p-hydroquinone. Muhammad Zahida,b*, Günter Gramppb, Asim Manshab,c, Ijaz Ahamad Bhattia, Sadia Asimb,c a Department of Chemistry & Biochemistry, University of Agriculture Faisalabad, Pakistan. b Institute of Physical and Theoretical Chemistry, Graz University of Technology, Graz/Austria c Department of Chemistry, GC University Faisalabad, Pakistan Supporting information (Supplementary data) 1. Absorption spectra of TCHQ in various solvents 1.1. TCHQ in Ether In low polarity solvents like ethers, the absorption spectra showed only one peak at 398 nm for different concentration as shown in the inset of Fig. S-1. Decrease in absorption intensity on dilution 3 0.20 Absorption spectra of TCHQ at very low conc. in diethylether have only one peak 398nm 2 Absorbance Absorbance 0.15 0.10 dilute solution 0.05 1 0.00 300 400 500 600 Wavelength (nm) 0 300 400 500 600 Wavelength (nm) Fig. S-1: Absorption Spectra of TCHQ in diethyl ether for different concentrations. 1 1.2. TCHQ in acetone and acetonitrile Two peaks were observed in the range of 300 to 600 nm for moderately polar solvents like acetone (Fig. S-2) and acetonitrile (Fig. S-3). The first peak is in the range of 390-400 nm and the second peak is around 505 nm. Decrease in absorption intensity on dilution 0.6 0.09 Absorbance Absorbance Most dilute solution of TCHQ 0.4 0.06 0.03 0.00 300 0.2 400 500 600 wavelength (nm) 0.0 300 350 400 450 500 wavelength (nm) 550 600 650 Fig. S-2: Absorption spectra of TCHQ in acetone for different concentrations. Two peaks were observed at 392 nm and 502 nm for various concentrations of TCHQ in acetonitrile as shown in Fig. S-3. This non-linear decrease in the absorption intensity at two different wavelengths (392 nm and 502 nm) has been shown clearly in the Fig. S-4 (a) and Fig. S-4 (b). 2 -5 Conc. 10 M 18.2 11.3 7.7 3.9 3.5 3.0 2.5 2.2 2.0 1.7 1.4 1.0 0.7 0.4 0.2 Absorbance 1.6 1.2 0.8 0.15 -5 Absorbance 2.0 Conc. 10 M 2.0 1.7 1.4 1.0 0.7 0.4 0.2 0.10 0.05 0.00 300 400 500 600 Wavelength (nm) 0.4 0.0 300 400 500 600 Wavelength (nm) Fig. S-3: Absorption spectra of TCHQ in acetonitrile at different concentrations. 2.0 (b) Absorbance (normalized) (a) Absorbance 1.6 1.2 0.8 392 nm 502 nm 0.4 0.9 0.6 0.3 392 nm 502 nm 0.0 0.0 0 3 6 9 12 15 18 -5 Conc. of TCHQ in MeCN (10 M) 0 4 8 12 16 -5 Conc. of TCHQ in MeCN (10 M) Fig. S-4: (a) Absorbance and (b) normalized absorbance of TCHQ in acetonitrile (MeCN) for different concentrations at 392 nm and 502 nm. 1.3. TCHQ in water and ethanol Two peaks of TCHQ were observed in water for most of the concentrations except very dilute ones (conc. < 1x10-5 M) as shown in Fig. S-5. Signal intensity at 460 nm has been decreased 3 rapidly as compared to the peak at 520 nm, with the decrease in concentration of the TCHQ in H2O as shown in Fig. S-6. Absorbance Concentration -5 16.6 10 M -5 5.6 10 M -5 2.8 10 M -5 1.8 10 M -5 0.9 10 M -5 0.5 10 M 2 Absorbance 0.3 3 0.2 Concentration -5 2.8 10 M -5 0.9 10 M -5 0.510 M 0.1 0.0 300 400 500 600 Wavenumber (nm) 1 0 300 400 500 600 Wavenumber (nm) Fig. S-5: Absorption Spectra of TCHQ in H2O at different concentrations. (a) 1.0 Absorbance Absorbance (normalized) 1.2 0.8 0.6 0.4 440 nm 540 nm 0.2 0.0 0 3 6 9 (b) 1.0 0.8 0.6 0.4 440 nm 540 nm 0.2 0.0 12 15 -5 Conc. of TCHQ in H2O (10 M) 18 0 3 6 9 12 15 18 -5 Conc. of TCHQ in H2O (10 M) Fig. S-6: (a) Absorbance and (b) normalized absorbance of TCHQ in H2O for different concentrations at 440 nm and 540 nm. Absorption spectra of TCHQ after adding a small amount of water (less than 2%) into the dilute solution of TCHQ (3 x 10-5 M) in absolute ethanol is shown in Fig. S-7. 4 0.5 Absorbance 0.4 0.3 Absolute ethanoll EtOH + H2O (<2%) 0.2 0.1 0.0 300 400 500 600 700 Wavelength (nm) Fig. S-7: Absorption spectra of TCHQ in absolute ethanol and ethanol containing water (< 2%). 2. Fluorescence emission spectra of TCHQ in different solvents excited at various wavelengths (a) Absorption spectrum of TCHQ in diethylether (b) Fluorescence spectra of TCHQ in diethylether excited at 390 nm, 430 nm & 460 nm. 0.8 Absorbace 8 2x10 0.4 0.2 (exc= 390 nm) 0.0 8 (exc= 430 nm) 1x10 (exc= 460 nm) 0 400 450 500 550 600 Wavelength (nm) 650 700 Normalized fluorescence intensity Fluorescence (counts) 0.6 750 1.0 300 400 500 wavelength (nm) 600 700 (c) Normalized fluorescence spectra of TCHQ in diethylether excited at 390 nm, 430 nm & 460 nm 0.8 (exc= 390 nm) 0.6 (exc= 430 nm) 0.4 (exc= 460 nm) 0.2 0.0 400 500 600 700 wavelength (nm) Fig. S-8. (a) Absorption, (b) fluorescence and (c) normalized fluorescence spectra of TCHQ in diethylether. The excitation wavelengths are 390 nm, 430 nm and 460 nm. 5 0.4 (a) Absorption spectrum of TCHQ in n-butyl ether Absorbace 8 1.5x10 0.2 300 8 1.0x10 exc= 390 nm 1.2 Normalized fluorescence intensity Fluorescence intensity (counts) (b) Fluorescence spectra of TCHQ in n-butylether excited at 390 nm & 460 nm wavelength 7 5.0x10 exc= 460 nm 0.0 400 500 600 700 Wavelength (nm) 400 500 Wavelength (nm) 600 700 (c) Normalized fluorescence spectra of TCHQ in nbutylether excited at 390 nm & 460 nm wavelength 1.0 0.8 exc= 390 nm 0.6 exc= 460 nm 0.4 0.2 0.0 400 500 600 Wavelength (nm) 700 Fig. S-9. (a) Absorption, (b) fluorescence and (c) normalized fluorescence spectra of TCHQ in nThe excitation wavelengths are 390 nm and 460 nm. butylether. (b) Fluorescence Spectrum of TCHQ in acetone excited at 515 nm. 0.3 (a) Absorption spectrum of TCHQ in acetone 8 1.0x10 Absorbace Fluorescence Intensity (counts) 8 1.5x10 7 5.0x10 0.2 0.1 400 0.0 500 550 600 650 700 500 600 wavelength (nm) 700 750 wavelength (nm) Fig. S-10. (a) Absorption, (b) fluorescence spectra of TCHQ in acetone excited at 515 nm. 6 1.0 (b) Fluorescence spectra of TCHQ in Acetonitrile excited at 390 nm & 515 nm wavelength 0.8 8 Absorbace Fluorescence Intensity 3x10 8 0.6 0.4 0.2 exc= 390 nm 2x10 (a) Absorption Spectrum of TCHQ in Acetonitrile 0.0 300 400 500 600 700 Wavelength (nm) exc= 515 nm 1.2 Normalized fluorescence 8 1x10 0 400 500 600 700 (c) Normalized fluorescence spectra of TCHQ in acetonitrile excited at 390 nm & 515 nm wavelength 0.9 exc= 390 nm 0.6 exc= 515 nm 0.3 0.0 300 Wavelength (nm) 400 500 600 700 Wavelength (nm) 800 Fig. S-11. (a) Absorption, (b) fluorescence and (c) normalized fluorescence spectra of TCHQ in acetonitrile. The excitation wavelengths are 390 nm and 515 nm. 0.20 (b) Fluorescence spectra of TCHQ in ethanol + water (<2%) excited at 460 nm ,515 nm & 550 nm wavelength Absorbace 7 7 0.15 0.10 5x10 exc= 460 nm 7 0.05 4x10 300 400 exc= 515 nm 7 3x10 exc= 550 nm 7 2x10 1.0 7 1x10 0 450 500 550 600 650 wavelength (nm) 700 500 600 700 wavelength (nm) 750 800 Normalized fluorescence intensity Fluorescence intensity (counts) 6x10 (a) Absorption spectrum of TCHQ in ethanol + water (<2%) (c) Normalized fluorescence spectra of TCHQ in ethanol + water (<2%) excited at 460 nm, 515 nm & 550 nm wavelength exc= 460 nm 0.8 exc= 515 nm 0.6 exc= 550 nm 0.4 0.2 0.0 450 500 550 600 650 700 750 800 wavelength (nm) Fig. S-12. (a) Absorption, (b) fluorescence and (c) normalized fluorescence spectra of TCHQ in ethanol + water (< 2 %). The excitation wavelengths are 460 nm, 515 nm and 550 nm. 7 (b) Fluorescence Spectra of TCHQ in DMSO excited at 515 nm, 570 nm & 670 nm wavelength Absorbace Fluorescence intensity (counts) 0.3 7 9.0x10 (a) Absorption spectrum of TCHQ in DMSO 0.2 0.1 exc= 515 nm 0.0 7 6.0x10 7 3.0x10 1.2 Normalized fluorescence intensity exc= 570 nm exc= 670 nm 0.0 500 550 600 650 700 Wavelength (nm) 750 800 300 400 500 600 Wavelength (nm) 700 800 (c) Normalized fluorescence spectra of TCHQ in DMSO excited at 515 nm, 570 nm & 670 nm wavelength 1.0 0.8 exc= 570 nm exc= 670 nm exc= 515 nm 0.6 0.4 0.2 0.0 500 550 600 650 700 750 800 Wavelength (nm) Fig. S-13. (a) Absorpti*on, (b) fluorescence and (c) normalized fluorescence spectra of TCHQ in DMSO. The excitation wavelengths are 515 nm, 570 nm and 670 nm. 8