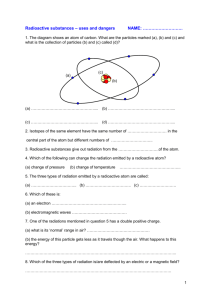
Nuclear Chemistry Worksheet: Alpha, Beta, Gamma & More
advertisement

Name ___________________________________ Period __________ Nuclear Chemistry Alpha, Becquerel, beta, binding force, cloud chamber, decays, fission, fusion, gamma, GeigerMüller tube, half life, isotopes, nuclear waste, nucleons, plutonium, radioactive, radiation, radioisotope, radiotherapy, stable, thermonuclear, transmutation. 1. Protons and neutrons are also known as__________________________________ . 2. Unstable nuclei are_________________________________ . 3. Alpha, beta and gamma area all types of nuclear _______________________________ . 4. __________________________________ particles are helium nuclei and __________________________________ particles are fast moving electrons. 5. Elements with different numbers of neutrons exist as ______________________________. 6. Particles in the nucleus are held together by a ___________________________________ . 7. Elements that don’t decay are ____________________________________ . 8. A _____________________________________ shows the path of ionising radiation. 9. The amount of radiation present in a sample can be measured using a ________________________ and counter. 10. __________________________ discovered radiation when uranium affected some of his photographic plates. 11. A _________________________________ is a radioactive isotope of an element. 12. _______________________________ is the change of one chemical element into another. 13. Radiation activity is measured in _________________________________or disintegrations per second. 1 Name ___________________________________ Period __________ 14. The __________________________ of a radioisotope is the time taken for its radioactivity to fall to Nuclear Chemistry half of its initial value. 15. _______________________________ can be used to treat cancer patients. 16. Many people oppose the use of radioactive materials for power generation because of the difficulties of disposing of_________________________________ . 17. _____________________________________ is a by-product of some nuclear processes. 18. _____________________________________ is the process of nuclei joining. 19. Nuclear fusion can result in a ____________________________________ explosion. Decide whether the following statements are true or false 1. Neils Bohr was the first person to come up with a theory on atomic structure. _____ 2. Electrons have approximately 2000 times the mass of a proton. _____ 3. All elements with an atomic number greater than 83 are radioactive. _____ 4. Unstable nuclei become stable by emitting radiation. _____ 5. The Periodic table lists all elements, both natural and synthetic. _____ 6. Irene and Frederick Curie produced the first radioactive isotope. _____ 7. The half lives of all radioactive elements are the same. _____ 8. In nuclear reactions, mass can be changed to energy. _____ 9. Radiation can be used to destroy abnormal cells. _____ 10. Radiation can be used to measure the thickness of sheets of metal. _____ 11. Nuclear power stations have only been constructed in the last ten years. _____ 12. Fusion is the process occurring in the Sun. _____ 13. Plutonium can remain radioactive for many thousands of years. _____ 14. Knowing the half-life of a radioisotope can help us determine the age of geological materials and artifacts. _____ 2