Nuclear Guided notes 201011
advertisement

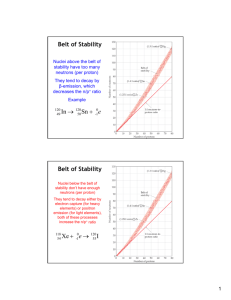
NAME: AP CHEMISTRY GUIDED READING ASSIGNMENT CHAPTER 21: NUCLEAR CHEMISTRY (SELECTED TOPICS) 25 points DIRECTIONS: Complete this packet and hand it in on the day of the chapter 21 test (due at the time the test is given). It is suggested that you work on a portion of it each night. Brief notes will be given in class; you can use this packet during class when notes are being given, but completing the packet does not replace the action of taking notes in class. It is expected that you will take notes during class, even if it is just adding to the material in this packet. Remember, the class notes are the primary source for test material. Class Problems from each section will be checked in class. A schedule for checking them will be provided at a later date. These problems do not have to be handed in with the guided notes packet. 21.1: RADIOACTIVITY 1. The term nucleon refers to which subatomic particles? 2. How is the mass number of an atom calculated? 3. How is the atomic number of an atom determined? 4. What are isotopes? 5. In general terms, what information is shown in a nuclear equation? 6. What type of particles are associated with each of the following types of radiation? Where in the atom is each particle from? What notation is used to represent the particle in a nuclear equation? -Radiation -Radiation -Radiation 7. When a nucleus undergoes spontaneous decay and a nuclear equation is written to represent it, what two things are conserved? 1 8. Write an equation to represent the alpha decay of Polonium-212. 9. What is a positron? 10. What occurs in a positron emission? Provide a sample equation. 11. What occurs in an electron capture reaction? Provide an example. CLASS PROBLEMS: # 2, 6, 8 21.2: PATTERNS OF NUCLEAR STABILITY 1. What is the strong nuclear force? 2. What is required for stability as a nucleus has an increasing number of protons? 3. In general, at what point does the belt of stability deviate from a 1:1 ratio? 4. What happens after Bismuth, element 83? 5. What type of decay do nuclei above the belt of stability undergo? Why? 6. What type of decay do nuclei below the belt of stability undergo? Why? 7. What type of decay do nuclei with atomic numbers greater than 84 undergo? Why? 8. What is shown in a radioactive series? 9. Explain the significance of the magic numbers. 10. What is special about nuclei with even numbers of protons and neutrons? CLASS PROBLEMS: # 12, 20 2 21.3: NUCLEAR TRANSMUTATIONS Read this section for some background information on equations. CLASS PROBLEMS: # 8, 23, 24, 25, 26, 63 21.4: RATES OF RADIOACTIVE DECAY 1. What is a first order kinetic process? 2. What is half-life as it pertains to a radioactive isotope? 3. In the rate law expression for radioactive decay, what does k represent? 4. What does the activity of a sample refer to? What are its units? CLASS PROBLEMS: # 29, 30, 31, 32, 33, 38, 68 21.7: NUCLEAR FISSION 1. In the process of fission, why must the incoming neutron move slowly? 2. What is a chain reaction? 3. Define the term critical mass. 4. What is the enriched uranium that is used in nuclear reactors? 5. What is the role of the control rods in a reactor? 3 6. Where does the heat produced in the core of a reactor go to? For what purpose? 7. Label this image of the basic design of a nuclear power plant: CLASS PROBLEM: # 53 4 21.8: NUCLEAR FUSION 1. In general terms, what occurs in a fusion reaction? 2. List a few of the possible products of a fusion reaction. 3. Are the products of the fusion reaction radioactive? 4. What are a few of the difficulties associated with fusion reactions? CLASS PROBLEMS: # 57 21.9: BIOLOGICAL EFFECTS OF RADIATION 1. What characteristic determines the penetrating power of radiation? 2. What is the difference between ionizing and nonionizing radiation? 3. What does ionizing radiation do to water in living tissue? CLASS PROBLEMS: # 55 & 56 5