Covalent properties past paper questions
advertisement
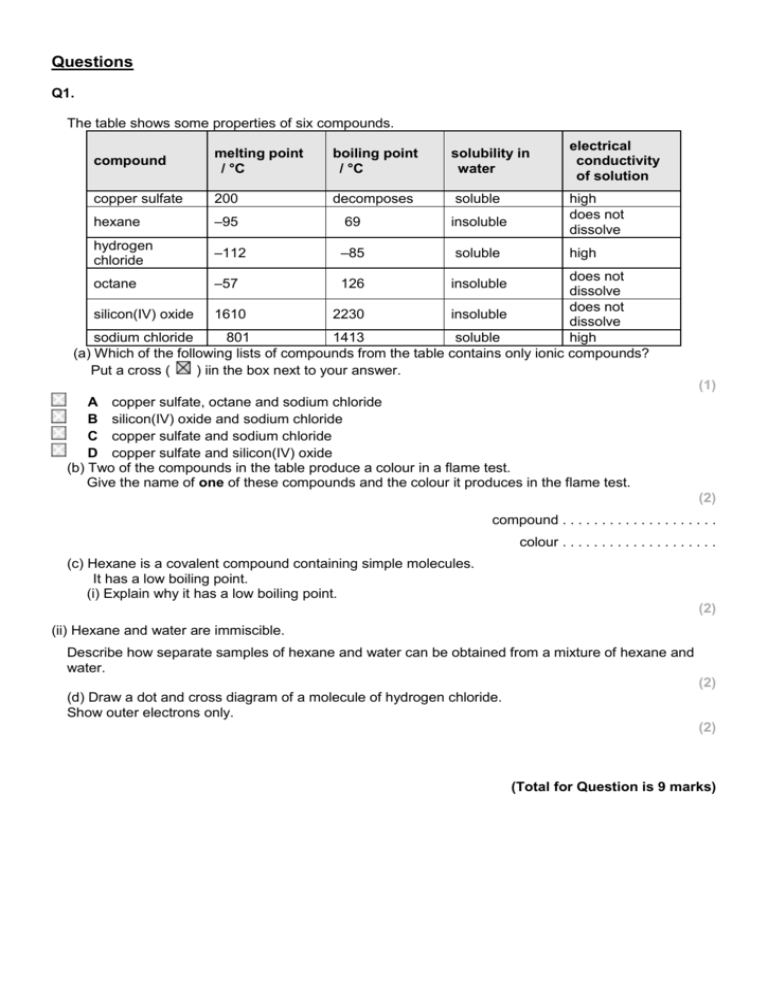
Questions Q1. The table shows some properties of six compounds. compound melting point / °C boiling point / °C solubility in water copper sulfate 200 decomposes soluble hexane –95 69 insoluble hydrogen chloride –112 –85 soluble electrical conductivity of solution high does not dissolve high does not dissolve does not silicon(IV) oxide 1610 2230 insoluble dissolve sodium chloride 801 1413 soluble high (a) Which of the following lists of compounds from the table contains only ionic compounds? Put a cross ( ) iin the box next to your answer. octane –57 126 insoluble (1) A copper sulfate, octane and sodium chloride B silicon(IV) oxide and sodium chloride C copper sulfate and sodium chloride D copper sulfate and silicon(IV) oxide (b) Two of the compounds in the table produce a colour in a flame test. Give the name of one of these compounds and the colour it produces in the flame test. (2) compound . . . . . . . . . . . . . . . . . . . . colour . . . . . . . . . . . . . . . . . . . . (c) Hexane is a covalent compound containing simple molecules. It has a low boiling point. (i) Explain why it has a low boiling point. (2) (ii) Hexane and water are immiscible. Describe how separate samples of hexane and water can be obtained from a mixture of hexane and water. (2) (d) Draw a dot and cross diagram of a molecule of hydrogen chloride. Show outer electrons only. (2) (Total for Question is 9 marks) Q2. Oxygen is a simple molecular, covalent substance. (i) The electronic configuration of oxygen is 2.6. Draw a dot and cross diagram for a molecule of oxygen, O2. Show the outer electrons only. (3) (ii) The boiling point of oxygen is –183 °C. Explain, in terms of the forces between the molecules, why oxygen has a very low boiling point. (2) .............................................................................................................................................. .............................................................................................................................................. .............................................................................................................................................. .............................................................................................................................................. Q3. Structures The table shows some properties of diamond and graphite. diamond graphite colourless crystals black, shiny solid very hard flakes easily does not conduct electricity conducts electricity (a) (i) Suggest why diamond and graphite might be expected to have similar properties. (1) .............................................................................................................................................. .............................................................................................................................................. (ii) By referring to its structure, explain why diamond is very hard. (3) .............................................................................................................................................. .............................................................................................................................................. .............................................................................................................................................. .............................................................................................................................................. .............................................................................................................................................. .............................................................................................................................................. (iii) By referring to its structure, explain why graphite flakes easily. (2) .............................................................................................................................................. .............................................................................................................................................. .............................................................................................................................................. .............................................................................................................................................. (b) Complete the sentence by putting a cross ( ) in the box next to your answer. Carbon dioxide is a gas at room temperature. A carbon dioxide molecule is a (1) A giant molecule that has covalent bonds B giant molecule that has ionic bonds C simple molecule that has covalent bonds D simple molecule that has ionic bonds (c) The atomic number of carbon is 6 and of fluorine is 9. Carbon and fluorine atoms are combined in a tetrafluoromethane molecule, CF4. Draw a dot and cross diagram of a tetrafluoromethane molecule. Show outer electrons only. (2) Mark Scheme Q1. Answer (a) (b) C : copper sulfate and sodium chloride copper sulfate (1) blue-green (1) or sodium chloride (1) yellow (1) colour mark consequential Acceptable answers Mark (1) allow blue or green or greenblue reject orange and yellow-orange (2) (c)(i) (c)(ii) on correct metal (compound) An explanation linking weak intermolecular forces /weak forces between molecules (1) little heat / energy needed to separate (molecules) (1) A description linking use separating funnel (1) run off lower layer / liquid / OWTTE (1) (d) shared pair in molecule (1) rest of molecule consequent on first mark (1) bonds / attractions in place of forces intermolecular forces between atoms / bonds loses 1st marking point any answer in terms of covalent or ionic bonding scores zero alternative description of separating funnel eg funnel with a tap at the bottom suitable labelled diagram burette allow layers / liquids to separate ignore fractional distillation Allow a diagram without labels for 2 marks any symbols shown must be correct for the 2nd mark allow any combination of dots and crosses for electrons wrong compound = zero marks (2) Acceptable answers ignore any inner electrons shown 3rd Mark is dependent on 2nd Mark (2) (2) Q2. Answer (i) (ii) electrons shared / between atoms (1) 2 pairs of/four electrons shared / between two atoms (1) 4 additional electrons on both oxygen atoms (1) An explanation linking the following second marking point is dependent on the first forces (between the molecules) are weak (1) therefore little (3) (2) intermolecular forces/bonds between molecules reject intramolecular force/covalent bond/ionic bond heat/energy needed to separate molecules/break these forces (1) Q3. Question Number (a)(i) Question Number (a)(ii) Question Number (a)(iii) Question Number (b) Question Number (c) Answer Acceptable answers Mark both (pure forms of) carbon / both giant molecular Answer Acceptable answers (1) Mark An explanation linking three of the following points • (every) carbon atom forms four bonds (1) • strong bonds / hard to separate atoms from lattice (1) • covalent bonds (1) • covalent bonds (1) Answer Acceptable answers (3) Mark An explanation linking the following • (in) layers (1) • weak forces between layers (1) Answer Acceptable answers (2) Mark Acceptable answers (1) Mark C Answer • four bonding pairs shown (1) • six non bonded electrons on each fluorine atom (1) (2)







