Unit 3 Study Guide-Chemical Bonds/Equations, Solutions, Acids
advertisement

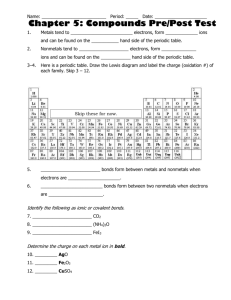
Physical Science Test #2 Study Guide Chemical Bonding - Chapter 19 1. Elements in the same group on the Periodic Table have the same number of _____________ electrons. 2. Metals are found on the ____________ side of the Periodic Table. ______________ are on the right side. ________________ are found along the zig-zag that separates metals and nonmetals. 3. What ending do we change binary compounds endings to? 4. What is unique about the noble gases? 5. An element is chemically stable when it obtains _______________ valence electrons. 6. Ionic bonds result from atoms ________________ electrons. Ionic bonds are usually formed between atoms of ______________ and nonmetals. 7. _______________ bonds result from atoms sharing electrons. These bonds are usually formed between _________________ and nonmetals. If the sharing of the electrons is equal then the bond is ______________ _______________. If the sharing of the electrons is ______________ then it is a polar covalent bond. 8. Write the chemical formula for the compounds that are made from the following elements. Then name the compound. Aluminum and Sulfur Calcium and Bromine 9. What is the name of this covalent compound? P2F5 10. Explain the difference between ionic and covalent bonds. 11. Complete the following table: Chemical formula Name P5O3 Magnesium Nitride heptanitrogen hexafluoride Type of compound (ionic or covalent) Chemical Reactions – Chapter 20 12. When balancing chemical equations, you are only allowed to adjust the ________________. 13. Name and describe the 4 types of chemical reactions. 14. Balance and classify the following chemical reactions. __CuO + ___Al ___Al2O3 + ___Cu ___Cl2 + ___NaBr → ___NaCl + ___Br2 ___BaCl2 + ___Na2 SO4 → ___N2(g) + ___H2(g) → ___NaCl + ___BaSO4 Type___________________ Type___________________ Type___________________ ___NH3(g) Type__________________ ___ Cu2O + _____ C ____ Cu +____ CO2 Type__________________ _____ Fe + _____ Cl2 Type__________________ _____ FeCl _____ Na + _____ HCl _____ NaCl + _____ H2 Type__________________ _____ KCl + _____ NaBr _____ KBr + _____ NaCl Type__________________ Solutions – Chapter 22 15. In a solution, the thing being dissolved is the _______________ and the thing doing the dissolving is the ______________________. 16. In saltwater, the solvent is the _____________________. 17. A solution that conducts electricity is an ___________________. 18. List three ways that you can increase the solubility of a solid in a liquid. 19. Explain the differences between unsaturated, saturated, and supersaturated solutions. 20. Compare a dilute and a concentrated solution. 21. What type of solution do you have if you dissolve 20 grams of KNO3 into 100 grams of water at 40 o C? 22. What type of solution do you have if you have 60 grams of NH4Cl into 100 grams of water at 90 o C? 23. What type of solution do you have if you dissolve 150 grams of KI into 100 grams of water at 10 o C? Acids and Bases – Chapter 23 24. A substance that produces hydrogen ions (H+) when dissolved in water is an ________. 25. An _________________ is something that changes color in the presence of an acid or a base. 26. A substance that has a pH = 13 and has a slippery feel, then we can say for sure that substance is a ______________. 27. If an acid only partially dissolves in water, then we can say it is a ________ acid. 28. Name four household acids and four household bases. 29. List common properties of acids and common properties of bases.