Chronic Opioid Therapy in Chronic Non
advertisement

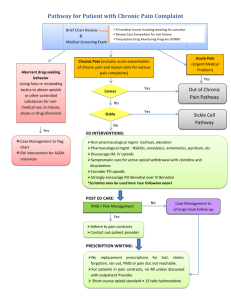
PARTNERSHIP HEALTHPLAN OF CALIFORNIA POLICY / PROCEDURE Policy/Procedure Number: MPRP4049 Lead Department: Health Services Policy/Procedure Title: Chronic Opioid Therapy in Chronic NonExternal Policy Cancer Pain Internal Policy Next Review Date: 10/1/2016 Original Date: 01/05/2012 Last Review Date: 10/1/2015 Applies to: Medi-Cal Healthy Kids Employees Reviewing Entities: IQI P&T QUAC OPERATIONS EXECUTIVE COMPLIANCE DEPARTMENT Approving Entities: BOARD COMPLIANCE FINANCE PAC CEO COO Approval Signature: Robert L. Moore, MD, MPH CREDENTIALING DEPT. DIRECTOR/OFFICER Approval Date: 10/1/2015 I. RELATED POLICIES: N/A II. IMPACTED DEPTS.: III. DEFINITIONS: N/A IV. ATTACHMENTS: A. Taken from, The Journal of Pain, Vol, 10 No 2 (February), 2009: pp 113- 130. Available at www.sciencedirect.com 1. Appendix B. Glossary 2. Appendix 4. Risk Assessment Tool (ORT) 3. Appendix 5. Risk Assessment Tool (D.I.R.E. Score) 4. Appendix 6. Sample Informed Consent form 5. Appendix 7. Sample Medication use Agreement 6. Appendix 8. Monitoring Tool — Pain Assessment and Documentation Tool (PADT), Progress Note V. PURPOSE: The purpose of this policy is to adopt and apply an evidenced based medicine approach in the guideline used in benefit design for the management of chronic opioid therapy (COT) for PHC member’s being treated for chronic non- cancer pain (CNCP). Treatment with chronic opioid medications comes with inherent concerns and risks to include; potential misuse, mortality, dependence and diversion. This policy further elaborates the role of the health plan, prescribers and member in helping to ensure that PHC members receive the highest quality and most cost effective management of their chronic non-malignant pain. BACKGROUND Partnership HealthPlan of California’s (PHC’s) aim is to consistently apply evidence-based medicine to the criteria used in clinical guideline development the area of chronic opioid usage in treatment of chronic pain management. In 2009, the American Pain Society and The American Academy of Pain Medicine published clinical guidelines for the use of chronic opioid therapy in chronic non-cancer pain. The plan has adopted this guideline as the standard of care for PHC members. http://www.jpain.org/article/PIIS1526590008008316/fulltext Page 1 of 4 Policy/Procedure Number: MPRP4049 Lead Department: Health Services ☒External Policy Policy/Procedure Title: Chronic Opioid Therapy in Chronic Non-Cancer Pain ☐Internal Policy Next Review Date: 10/1/2016 Original Date: 01/05/2012 Last Review Date: 10/1/2015 ☒ Healthy Kids Applies to: ☒ Medi-Cal ☐ Employees VI. POLICY / PROCEDURE: A. Role of the Health Plan 1. PHC identifies its members being treated with COT for CNCP through following methods; administrative data review, prior authorization requests beyond current benefit design, prior authorization renewal, provider input or other reasonable means of referral. For a request to meet medical necessity criteria the request must be accompanied by the supporting documents either from the attached appendices taken from the American Pain Society or by a like document submitted by the requesting physician as outlined in the procedure. a. Ongoing pain management is part of the scope of primary care and is to be managed and coordinated by the primary care practitioners. The plan provides access to pain management referral specialists (in person or by phone) to assist the primary care physicians in management of complex patients. Pain management specialist involvement may be requested and required when there is high dose (greater than 120mg of morphine sulfate or equivalent use/day) escalation and/or transition to a formulary preferred opioid. The role of the specialist is to evaluate the member and provide consultative assistance and appropriate specialty interventions to the member. The plan provides regular education on best practices in chronic pain management to the network. b. The Plan provides education on best practices in chronic pain management at academic detailing visits. c. The plan assists the prescribers by identifying outliers such as members utilizing high dose opioids, members with known compliance problems, members receiving controlled substances from multiple prescribers and shares this information with the prescriber. d. The plan provides tools to prescribers such as model pain contracts, sample risk assessment tools, restricted drug status and urine drug screening standards to assist the prescribers in safely prescribing opioids to PHC members. e. The plan establishes prior authorization criteria, and quantity limits for certain controlled substances through its Pharmacy and Therapeutics Committee and Physicians Advisory Committee to maximize the cost effective and highest quality of care to its members. B. Role of the Prescribers 1. As prescribers of controlled substances for PHC members, the treating practitioners have a unique role and set of responsibilities to ensure that members receive the highest quality of care and service. Specific responsibilities of prescribers include: a. Review and be knowledgeable about PHC’s standards of care, prior authorization criteria and the APS clinical guideline for management of non-malignant pain. b. Follow PHC’s prior authorization processes for use of non-formulary medications and submit documentation from the medical record to support the requests. When evidence of failure of a formulary medication is submitted supporting documentation must included evidence of a good faith trial and appropriate management of side effects before a non-formulary medication is considered. c. Utilize a medication use agreement (sometimes called a pain contract) for all PHC members receiving controlled substances for three months or longer. See attached for a model contract d. Order and evaluate a urine drug screen for all members on chronic opioid therapy at least yearly e. As long as available, obtain and review a Patient Activity Report (PAR) through the States Department of Justice database known as Controlled Substances Utilization Review and Evaluation System (CURES) at least yearly. The web site for signing up for on-line CURES reporting is http://ag.ca.gov/bne/cures.php f. Minimum three (3) member visits/year. Page 2 of 4 Policy/Procedure Number: MPRP4049 Lead Department: Health Services ☒External Policy Policy/Procedure Title: Chronic Opioid Therapy in Chronic Non-Cancer Pain ☐Internal Policy Next Review Date: 10/1/2016 Original Date: 01/05/2012 Last Review Date: 10/1/2015 ☒ Healthy Kids Applies to: ☒ Medi-Cal ☐ Employees g. Provide education to PHC members about the appropriate goals of pain management therapy and provide ancillary services as appropriate. h. Evaluate all PHC members undergoing treatment for chronic pain for behavioral health conditions such as depression regularly at each visit and provide behavioral health treatment and/or referral as necessary. C. Role of the member 1. Each PHC member has an important role and set of responsibilities with respect to their management of chronic pain. a. The member is responsible for complying with all of the requirements of the pain contract. Any violation of the contract may be ground to discontinue utilizing a safe tapering process of the controlled substance. b. Understand and be informed about the management of chronic pain. c. Actively engage in self-management of the chronic pain and focus on improving the member’s ability to function within the patient’s environment. d. Address behavioral health conditions as recommended by the patient’s treating practitioners. D. Opioid use and Dependence 1. All opioid use can lead to tolerance, dependence and addiction. Use of agents such as methadone and buprenorphine / naloxone (Suboxone) for withdrawal and addiction treatment is limited to only physicians with special state and federal licenses. Methadone can be prescribed by any physician with a Drug Enforcement Administration (DEA) license for treatment of acute or chronic pain and only limited use for temporary maintenance or detoxification when an addicted patient is admitted to acute care facilities. Buprenorphine, buprenorphine/naloxone use is limited to physicians who meet certain qualifying requirements from US Food and Drug Administration (FDA) and Secretary of Health and Human Services (HHS) and have been assigned a unique identification number. Buprenorphine drug products are non-capitated drugs for PHC Medi-Cal line of business and qualified prescribers will need to obtain Treatment Authorizations from State Medi-Cal. VII. REFERENCES: A. Opioid Treatment Guidelines. Clinical Guidelines for the Use of Chronic Opioid Therapy in Chronic Noncancer Pain. Chou, et. al., The Journal of Pain, Vol 10, No 2 (February) 2009: pp 113-130. VIII. DISTRIBUTION: A. PHC Department Directors, B. PHC Provider and Practitioner Manuals, C. SharePoint IX. POSITION RESPONSIBLE FOR IMPLEMENTING PROCEDURE: A. Pharmacy Services Director X. REVISION DATES: Medi-Cal 01/16/14; 10/1/2015 Healthy Kids 01/16/14; 10/1/2015 Page 3 of 4 Policy/Procedure Number: MPRP4049 Lead Department: Health Services ☒External Policy Policy/Procedure Title: Chronic Opioid Therapy in Chronic Non-Cancer Pain ☐Internal Policy Next Review Date: 10/1/2016 Original Date: 01/05/2012 Last Review Date: 10/1/2015 ☒ Healthy Kids Applies to: ☒ Medi-Cal ☐ Employees PREVIOUSLY APPLIED TO: PartnershipAdvantage: MPRP4049 - 01/05/2012 to 01/01/2015 XI. POLICY DISCLAIMER: A. In accordance with the California Health and Safety Code, Section 1363.5, this policy was developed with involvement from actively practicing health care providers and meets these provisions: 1. Consistent with sound clinical principles and processes; 2. Evaluated and updated at least annually; 3. If used as the basis of a decision to modify, delay or deny services in a specific case, the criteria will be disclosed to the provider and/or enrollee upon request. B. The materials provided are guidelines used by PHC to authorize, modify or deny services for persons with similar illnesses or conditions. Specific care and treatment may vary depending on individual need and the benefits covered under PHC. Page 4 of 4