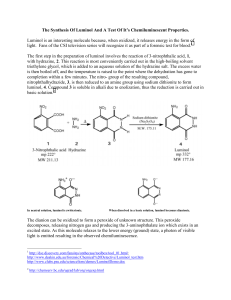
luminol1
advertisement

o Used to find latent blood & fluid stains. o Glows in the dark on contact with blood. o The Luminol test for blood was developed by Walter Specht of Germany in 1937. oLuminol is sensitive in the parts per million range even for blood years old. Thus, If you have 1 drop of blood in 999,999 drops of H2O, Luminol will glow!! Practical Uses at The Scene Luminol usually finds use if there’s no obvious blood evidence in violent or sexual crimes. 1. Luminol is mixed fresh. 2. Investigators darken the room. 3. The room is sprayed with Luminol mixture. 4. Areas with blood or fluid evidence glow blue-green. Glow time is important because different materials have different glow durations. Luminol Chemistry 1. Luminol crystals are mixed in water buffered to pH 10.5. 2. Hydrogen Peroxide is added & the reaction starts. 3. Addition of a catalyst (Iron in blood) speeds up the reaction (giving enough light to see!) 4. So you’re actually detecting iron catalyst! Luminol is not radioactive! (in case you wondered) How Luminol Can Help Cold Cases •Footprints can link a suspect to a crime. •Trace amounts of blood on a carpet may lead to a pool underneath. • Blood spray patterns can be determined (Useful for trajectory and weapon identification). • Was the body dragged? If evidence is found, it’s videotaped and photographed using special techniques. Evidence is collected. Drawbacks o Luminol uses up blood during its reaction and thus destroys evidence! o Other popular crime scene materials like BLEACH and Metals react – Further blood testing is a must! The glow of metals fades quickly, but the glow of blood lasts longer. Experts can tell if they have blood or something else. Safety o Luminol irritates the eyes, skin and lungs. o Blood can be contaminated with diseases. o Wear gloves, eye protection and a surgical gown to protect against these dangers. o Decontaminate yourself & your gear when you’re done with dilute bleach.