EXPERIMENT 27 LMINOL SYNTHESIS OF A
advertisement

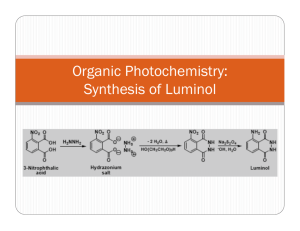
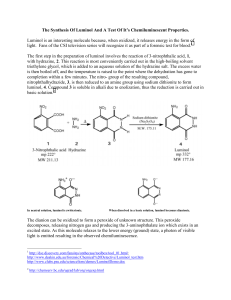
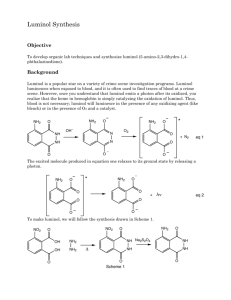
EXPERIMENT 27 LMINOL SYNTHESIS OF A CHEMFLUMINESCENT SUBSTANCE Chemical changes are accompanied by energy changes. In most cases, energy change takes the form of heat transfer, and chemical reactions are classified as exothermic or endothermic. In a few rare instances, the energy transfer occurs by emission of light. This phenomenon is called chemi- luminescence. The particular compound to be prepared in this case is called "luminol", 3-aminophthalhydrazide. When a basic solution of this compound is oxidized, a brilliant blue-green light is emitted. Luminol is an excellent example of chemiluminescence. The synthesis of luminol involves several stages. The starting material, 3nitrophthalic acid (1) is first reacted with hydrazine to form 3nitrophthalhydrazide (2). The 3-nitrophthalhydrazide is treated with sodium hydrosulfite (Na2S2O4), a mild reducing agent to form 3-amino-phthalhydrazide (3). This compound is luminol. NO2 NO2 O NH2 COOH N + NH2-NH2 N COOH (1) (2) H Na2S2O4 O N N H O (3) H H O When luminol is placed in an alkaline solution, two hydrogens are removed, forming water and a resonance-stabilized dianion (4) is formed. This dianion is oxidized by a combination of hydrogen peroxide and potassium ferricyanide to 3- aminodiphthalate (5). This oxidation process is accompanied by emission of N2 and a photon of light. NH2 O N H N (3) O NH2 NH2 O - N N- + 2 OH- H (4) COO- K3Fe(CN)6 N2 + Light + COO- H2O2 O (5) The light-producing reaction is best seen in a darkened room. PROCEDURE I. PREPARATION OF SOLID LUMINOL: Set a small beaker of water to boil on a hotplate while you carry out the rest of the procedure. To a round-bottomed boiling flask or small Erlenmeyer flask add 400mg of 3-nitrophthalic acid and 0.8mL of 8% aqueous hydrazine solution. CAUTION!! Heat this mixture on a sandbath until all of the solid dissolves. Add 1.2mL of triethylene glycol and a boiling chip. Clamp the flask in place with its base buried deep in the sandbath. Insert and clamp a thermometer in place so that the temperature of the boiling mixture can be constantly monitored. Boil the solution vigorously. At first, the boiling point will be at about 1100C, then, once all the excess water has been driven off, there will be a sharp increase in the temperature to approximately 2150C. Maintain the temperature between 215 and 2200C for two minutes by heating intermittently. This can be achieved by alternately lifting the flask above the sand and then replacing it. After this two-minute period, remove the flask from the heat and cool the solution to about 100 0 C. At this time, crystals of the product may begin to form. Pour in 6mL of the hot water which you had set to boil at the beginning of the procedure. Cool the flask in cold water and stir. Then collect the light-yellow, granular compound 3nitrophthalhydrazide by suction filtration. Transfer this solid back to the flask in which it was prepared. Add 2mL of 10% sodium hydroxide solution and stir. A red-brown solution should form. To this solution, add 1.2g of sodium hydro-sulfite. Wash the solid down the walls of the flask with a little water. Heat to the boiling point, stir, and maintain boiling for about 5 minutes. Then add O.8mL of glacial acetic acid, cool the flask in a beaker of cold water and stir. Collect the resulting light-yellow precipitate of Luminol by suction filtration. II. PREPARATION OF STOCK SOLUTIONS: Dissolve the moist Luminol prepared in the first part of the procedure in a mixture of 4mL of 10% sodium hydroxide solution in 36mL of water. THIS IS STOCK SOLUTION "A". To prepare STOCK SOLUTION "B" mix 4mL of 3% potassium ferricyanide solution, 4mL of 3% hydrogen peroxide solution and 32mL of water. III. THE CHEMILUMINESCENT REACTION: Dilute 5mL of STOCK SOLUTION "A" with 35mL of water. In a dark room, pour this dilute solution and all of SOLUTION "B" simultaneously into a suitable flask. Swirl the flask and gradually add further small quantities of sodium hydroxide and potassium ferricyanide solutions in order to increase the brilliance. NOTE: BEFORE CLEANUP. PUT ANY EXCESS STOCK SOLUTION "A" IN REAGENT ( BOTTLE AT FRONT OF CLASSROOM. EXPERIMENT 27: Report and W orksheet LOMINOL - SYNTHESIS OF A CHEMILUMINESCENT SUBSTANCE Student Name:_______________________________ Student Number:_____________________________ Day:_____________ Date:____________ DATA OBSERVATIONS Step 1: Preparation of 3-nitrophthalhydrazide. Step 2: Reduction of 3-nitrophthalhydrazide to luminol. Step 3: Oxidation of luminol. QUESTIONS 1. W hat is the EQUATION for the reaction of 3-nitrophthalic acid with hydrazine? What KIND of reaction is this? 2. What is the EQUATION for the reduction of 3-nitrophthalhydrazide to luminol? 3. Draw at least TWO RESONANCE STRUCTURES for the dianion formed when luminol is treated with NaOH. 4. What is the EQUATION for the oxidation of the dianion of luminal?