File
advertisement

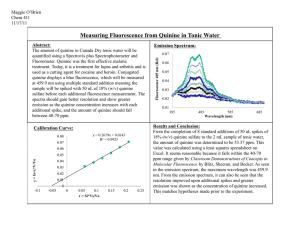
Ovapositioning Preference with Quinine Hydrate by Adult Female Drosophila Eric Schmit Victoria Rottmann Mercedez Kennedy Department of Sciences, Loras College 1450 Alta Vista St. Dubuque, IA 52001 Abstract: The study of ovapositioning is often done in Drosophila but they often test whether a fly will choose the best medium compared to a lesser one, or whether they prefer a similar medium to what they were reared in themselves compared to another of similar qualities. The procedure performed test both of these theories against each other, and found that the larger role is what the drosophila was reared in itself and not optimal nutrition, however, much more research is found on what is the best nutrition selection then how a drosophila makes this selection. Introduction: By rearing multiple generations of Drosophila on a mixture of standard lab quality fly food and small levels of quinine hydrate, we had hope to raise a generation of flies that could tolerate low levels of quinine, which is highly toxic to species such as drosophila. We would then proceed to test this generation for ovapositioning preference, or the choice of medium used to lay their offspring, for whether they would choose a medium with or without the familiar quinine concentrations present to lay their eggs, with all else held constant (Yang, Belawat). Because quinine is toxic to drosophila, the medium without any levels of quinine would be a much higher quality medium. To create a control variable, several generations of drosophila were reared under the exact same conditions, except this group had no levels of quinine hydrate in their food. This control group would be given the exact same amounts of food, water, light, temperature and time for each generation so that other variables would not play as much as an issue in our results. Previous research studies has shown that the female drosophila will lay their offspring in the best quality medium available (Sheeva), while other studies show that female drosophila will select a medium close to what they were reared on themselves(Yang, Belawat). With this knowledge and the must more present research that data that female drosophila choose the better quality egg laying medium, we formed our hypothesis that the female drosophila will choose the high quality medium without the quinine levels, rather than the medium with the quinine levels present. Methods: This research was done in several stages. Our first stage was to create four different variables or concentrations of quinine hydrate to be used to start our generations of drosophila. We used eight glass bottles designed for rearing the drosophila, dedicating two for each level of quinine present. One of these levels would be our control, and would only receive 9 grams of Carolina Pre-made fly food and 30ml of deionized water each and were appropriately labeled. The next two bottles received the same 9 grams of food, but instead received 30 ml of .01 molar quinine hydrate. Due to the solution being a paste rather than a liquid, 10 more milliliters of deionized water were added to allow to food medium to set. The next two bottles were given 9 grams of fly food each, and were given 30 ml of .05 molar quinine hydrate. The last two bottles were given 9 grams of fly food each and mixed with 30ml of .005 molar quinine hydrate. Each bottle was allowed to set until the food formed an opaque pasty gel on the bottom. After the food had set, groups of 40-60 drosophila melanogaster were added to each bottle, capped with a sponge cork to allow quality air flow, and placed in an incubator set at 20 degrees Celsius, each bottle being at the same shelf level to receive equal lighting. The control group was the first to rear its first offspring generation. When the larva matures in the food medium from the previous generation, it climbs up the side of the bottle and turns a dark brown color. This means that that larva is readying to hatch and become a young drosophila. When more than 25 of these dark larvas occur, which took the control bottles 10 days, the bottle was emptied of all adults which were no longer needed in this research. The control bottles remained untouched from that point for 12 hours, allowing a new generation of drosophila to hatch. These were immediately collected in bottles with the exact same food and water specifications, and placed back in the incubator for five days. To test ovapositioning, two chambers were created that consisted of large two quart sized tubberware containers, with several small ventilation holes on the top. The containers were flipped so that the lids became the bottom. On the lids, we placed six egg laying mediums each. Based on research, the best egg laying medium seemed to be an agar material made by boiling 77.5ml water, 20 ml Welches pure grape juice (no sugars added), and 1 gram of agar. After the substance reached a boil, we removed it from heat, and let it cool until it reached 50 degrees Celsius. At this point, 0.5 ml glacial acid, 1.0 ml 95 percent Ethyl Alcohol, and one drop of blue food coloring to help the eggs stand out. The solution was poured into six empty petri dishes. To create a medium with quinine, we repeated the above steps, but used only 67.5 ml water and 10ml of .01 molar quinine hydrate, to achieve a desirable amount of quinine presence that would be evident to the drosophila, but not lethal. To test ovapositioning, 24 female drosophila were selected from each bottle and placed in a different container, placing 4 on each medium. The flies would be subjected to ether for a short term to knock them out, and the best of the females would be selected and placed four on a dish. When the females awake in the breeding chamber, they are free to fly to the medium of their choice to lay their eggs. The entire container is placed in a separate incubator at 20 degrees Celsius for 12 hours for this process. After the 12 hour period, the containers are removed, flies released, and the eggs are counted and recorded for each dish under a dissection microscope. The bottles containing .05 and .005 molar quinine levels achieved enough larvas to hatch at the same time, seven days later than the control bottles on day 17. These bottles were also then emptied of all adult drosophila, and sat untouched for 12 hours until the newly hatched drosophila we removed, and placed in new bottles with the same specifications. Due to very low levels of surviving drosophila, each quinine level group was placed in one bottle. Now .05 and .005 levels only had one bottle. The .01bottles had yet to achieve any larva ready to hatch, and the larva present appeared to be much smaller than normal healthy drosophila. After five days passed, the two bottles containing the new generation of quinine reared drosophila were ready to be tested for ovapositioning. Again, the two containers were set up, one for each level of quinine that the drosophila were reared one. Both containers had the quality medium used in the control test, and the same concentration of the quinine medium as used in the control test. The containers are treated exactly as the control groups for 12 hours and the offspring are counted. Results: The presence of Quinine affected the growth and development of the drosophila. This caused the number of offspring of each level of quinine to drop as levels increase. The .01 group actual never succeeded at producing larva that matured enough to produce flies, and the larva was always much smaller than the normal larva. The eggs laid by both the .005 and .05 in the mediums were noticeably smaller then flies not reared on quinine, and they also seemed to have laid larva rather than eggs more often than the control groups. This effects were not found in any previous research. The results for the ovapositioning procedure are shown per group: Control Group (48 flies) Quality Medium egg counts Quinine Medium egg counts 21 8 28 0 12 0 12 3 6 1 47 0 .05 Quinine Reared Group (24 flies) Quality Medium egg counts Quinine Medium egg counts 2 6 0 3 2 14 .005 Quinine Reared Group (24 flies) Quality Medium egg counts Quinine Medium egg counts 18 11 3 21 5 10 Without even looking at any statistical data, the two groups reared on quinine seemed to have preferred laying their eggs in quinine-medium. When entered on statistical software, SPSS, a Univariate Analysis of Variance proves with a significance value of .011 that there is a difference between the different groups. (Figure 1) Figure 1: Tests of Between-Subjects Effects Dependent Variable:egg_count Source Type III Sum of Squares df Mean Square F Sig. 1402.958a 5 280.592 3.517 .022 Intercept 1793.067 1 1793.067 22.476 .000 Container 217.125 2 108.562 1.361 .282 32.267 1 32.267 .404 .533 925.792 2 462.896 5.802 .011 Error 1436.000 18 79.778 Total 5101.000 24 Corrected Total 2838.958 23 Corrected Model Quinine_Presence Container * Quinine_Presence a. R Squared = .494 (Adjusted R Squared = .354) Figure 1. The bolded line, Container*Quinine Presence test for in-between group variance, and shows that there is a significant difference between all three of the groups. The F-Value of 5.802 being the highest means that Groups separated by Quinine levels is the main source of variation. Because there is a significant difference between each container, it must be broken down to show the variance of each group from each other. This can be done by a Tukey PostHoc test on SPSS. (Figure 2) Figure 2: Multiple Comparisons egg_count Tukey HSD (I) Container (J) Container Difference (I-J) Control .05 M reared .005 M reared 95% Confidence Interval Mean Std. Error Sig. Lower Bound Upper Bound .05 M reared 7.0000 4.46592 .285 -4.3978 18.3978 .005 M reared .1667 4.46592 .999 -11.2311 11.5644 Control -7.0000 4.46592 .285 -18.3978 4.3978 .005 M reared -6.8333 5.15680 .400 -19.9943 6.3277 Control -.1667 4.46592 .999 -11.5644 11.2311 .05 M reared 6.8333 5.15680 .400 -6.3277 19.9943 Based on observed means. The error term is Mean Square(Error) = 79.778. Figure 2: The above table shows the significance of each group compared to other groups. None of the groups are significantly related to one another The best way to visually see the trend that the drosophila follow as quinine levels increase is through a simple graph of the data of mean egg and the presence of quinine is the egg laying medium. (Figure 3) Discussion: Based on our findings, we cannot reject our null hypothesis that the female drosophila will not select the best medium available to rear their offspring on. Instead, we could further our research by testing a new hypothesis that the female drosophila are most likely to rear offspring on a medium that they were reared on themselves. Possible sources of error came from not having enough flies due to the toxicity of quinine to drosophila, and the negative effects that it had on the surviving flies. There is much research that suggest many factors why a female drosophila may select an egg laying medium, and it is possible that too many factors play a role to get a clear, significant choice. Acknowledgments: For the research, many thanks is given to Dr. Fred Schnee of Loras College for helping to set up the lab procedure and provide necessary equipment. Dr. Schnee continuously checked up on the research and had given much insightful information. Thanks also go out to Melissa Herman for explaining how to properly and efficiently proceed with the processed involved with rearing the drosophila. Literature Cited: Lee, K. P., Simpson, S. J., & Taylor, P. W. (2007). Lifespan and reproduction in drosophila: New insights from nutritional geometry . National Academy of Sciences, (Sept), 109. Sheeva, B., Madhyastha, A., & Joshi, A. (1998). Oviposition preference for novel versus normal food resources in laboratory populations of drosophila melanogaster . Journal of Biosciences, 23(2), 93-101. Sheeva, B., Rajamani, M., & Joshi, A. (1999). Research communications - bimodal distribution of oviposition preference for a novel food medium in drosophila melanogaster . Current Science, 9, 77-80. Vijendravarma, R. K. (2009). Effects of parental larval diet on egg size and offspring traits in drosophila. (Master's thesis, University of Lausanne, Lausanne, Switzerland ). Yang, C. H., Belawat, P., Hafen, E., Jan, L. Y., & Jan, Y. N. (2008). Drosophila egg-laying site selection as a system to study simple decision-making processes.