Chemistry Part 1 Test
advertisement

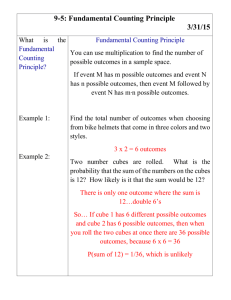
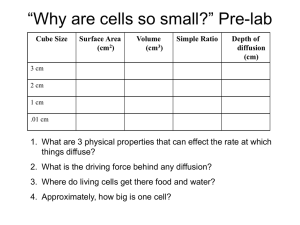
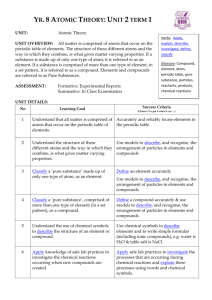
Chemistry Part 1 Test LT.C.1 I can explain the differences between an element and a compound. 1) A. Identify the following as a compound or element: 1) O2 2) H2O B. Explain why some molecules are not compounds. C. Describe why Ti is an element and not a compound. LT.C.2 I can classify a substance as an element or compound. 1) Which of the following is an element? A) O B) CO2 C) H2O D) HCl 2) Identify the following as an element or a compound: C6H12O6 3) Identify the following as an element or a compound: H2 LT.C.3 I can compare physical and chemical properties. 1) A. What are physical properties? B. What are chemical properties? C. List three physical properties of matter and one chemical property of matter. LT.C.4 I can explain density as a physical property. 1) What is the density of an object that has a mass of 60g and a volume of 10mL? a) 6mL/g b) 6g/mL c) 0.6mL/g d) 0.6g/mL 2) If a cube that had a density of 5g/mL was broken into a larger cube and a smaller cube what would be true about the two pieces? a) larger cubes density > smaller cubes density b) larger cubes density <smaller cube density c) larger cubes density=smaller cubes density d) none of the above are true LT.C.5 I can explain how density can be used. 1) Choose the object that would be the best to use to make a raft: a) object weighing 1 pound with a density of 3.27g/mL b) object weighing 0.5 pound with a density of 13.7g/mL c) object weighing 200 pounds with a density of 1.5g/mL d) object weighing 125 pounds with a density of 0.876g/mL LT.C.6 I can organize elements and compounds based on their properties. 1) Here are six elements. Organize them into two groups based on their properties. Explain how you separated the groups. Element Density (g/mL) React to O2 Conductivity Malleable Element A Element B Element C Element D 1.23 1.45 7.32 7.1 Yes No No No Yes No No No Yes No Yes No State of matter under normal conditions Solid Gas Liquid Gas Element E Element F 2.2 2.6 Yes No Yes No No Yes Solid Solid LT.C.7 I can infer real life applications for a substance based on properties. 1) Determine whether substance A or substance B is better for an outdoor statue in an area with high amounts of acid rain. Substance A= a type of stone, hard, very dense (>5 g/mL), reacts and dissolves in acid. Substance B= a type of stone, hard, fairly dense (>3g/mL), does not react with acid. 2) Infer a real life application for a material with the following properties: Shiny, very conductive of both heat and electricity, very malleable, very ductile, non-reactive, non-flammable, density= 3.74g/mL. LT.C.8 I can explain solids, liquids, and gases at the molecular level. 1) Draw a diagram of the atoms of a solid, liquid and gas. Below each be sure to include a short description of what is occurring. 2) Choose two different phase changes and explain what is happening with the energy in the substance and to the atoms at the molecular level. LT.C.9 I can explain how all matter is composed of different combinations/amounts of 92 naturally occurring elements. 1) Explain how it is possible for all matter on Earth to be composed from only 92 elements. LT.C.10 I can read the periodic table. 1) Using the periodic table determine how many protons are in an atom of C. a) 12 b) 6 c) 1 d) 0 2) Using the periodic table determine how many neutrons are in an atom of O. a) 8 b) 0 c) 16 d) 1 3) Using the periodic table write out the atomic mass of Cl. LT.C.11 I can use patterns of reactivity to determine real life applications for elements and compounds. 1) Given the fictional periodic table family below, predict a real life application for element X. a) electric wiring b) used in matches c) raft material d) insulated cup element T Density (g/mL) 1.2 U 1.9 V 2.5 W 3.4 X color conductivity React to fire Very light brown/dull Light brown/semidull Very high Yes low Darker brown/shiny Very low React to water Yes malleability Only after long exposure Yes Yes No Only after long exposure No Yes Yes Yes Yes