Properties of Ionic lattices - All Hallows Catholic High School

Properties of Ionic lattices
An ionic lattice is the regular arrangement of oppositely charged ions attracting each other that holds an ionic substance together.
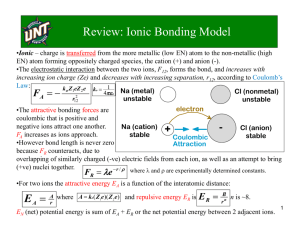
The diagram shows the bonding in sodium chloride (a salt) Na
The green balls represent Chloride Cl -
+ Cl - .
ions, the blue balls represent the sodium Na + ions.
The lines between the balls represent the forces of attraction (electrostatic forces) holding it together.
You should be able to describe / label the structure of an ionic lattice
The electrostatic forces of attraction between the ions are very strong and so ionic substances have high melting and boiling points because….. it takes a lot of energy to vibrate the ions hard enough to overcome the strong electrostatic forces and break the ions away from each other.
You should be able to explain why ionic substances have high melting and boiling points.
When….. the ionic subtance is dissolved in water or melted (molten) the lattice (regular arrangement) breaks down and the ions are free to move around.
Ionic substances are good conductors of electricity because….. the ions are charged. The ions (when molten or dissolved in water) can move and carry the charge and allow a current to flow.
You should be able to explain when & why ionic substances can conduct electricity and allow current to flow through them.