INSTRUCTIONS TO AUTHORS FOR THE PREPARATION
advertisement

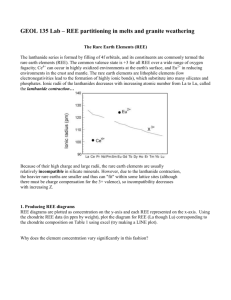
Significant reduction in acid consumption in rare earth extraction from ferrocarbonatite (Montviel project) Pouya Hajiani Geomega (phajiani@geomega.ca) ABSTRACT The viability of an extractive metallurgy process of rare earth elements (REEs) to produce an intermediate mixed concentrate depends upon a low-cost simple flowsheet. It has been known that regardless of the mineral, the acid consumption is intimately associated with the operational cost (OPEX) of any REE project, particularly when the initial REE grade is low. Beside acid, the other reagents intake in the method impact the OPEX. This makes minimizing acid consumption in an REE method a critical priority. The acid consumption in different projects has been reported to be equivalent of 100 kg to 1000 kg HCl100% per ton of ore (Average: 500 kg/T HCl 100%). This value is strongly correlated with the grade of REE in the ore and therefore in concentrate. The flotation concentrate of Montviel project contains about 3% rare earth oxides (REO), mostly occur in bastnaesite mineral. A typical flotation concentrate (FC) composition is given in Table 1 below: Element Elemental % Fe 21.53 Ba 6.52 Sr 2.35 Ca 6.65 Mg 3.77 Mn 2.76 Si 1.12 Nb 0.21 REE 3.99 High acid consumption in a conventional extraction circuit is due to the presence of other dissolvable elements, mainly iron and gangue minerals besides REEs in the concentrate. In order to save the acid, the alkali earth metal, originated from gangue minerals was removed prior to REE leaching. Moreover, operating condition of leaching reactor is optimized to leave iron behind and to leach REE selectively and effectively. Consequently the acid consumption per ton of ore drops significantly compare to other methods. For instance, REE extraction form Montviel FC (Table 1 above) requires 293 kg HCl (100%)/MT ore to attain +99% REE recoveries by conventional HCl leaching. The elemental acid consumption distribution is demonstrated in FIG. 1a. FIG. 1a shows that 88% of the acid is used to elute iron and gangue minerals and only 5% of HCl extracts REEs. Pretreatment of FC removes the gangue minerals without reagent consumption or imposing significant energy penalty. The acid consumption for +99% REE extraction drops to 191 kg HCl (100%)/MT ore after this treatment with elemental distribution shown in FIG. 1.b. At this point, iron is the most important acid consumer (67%). Optimization of leaching reactor provides selective leaching of REE and the acid consumption drops to 63.2 kg HCl (100%)/MT ore to recover +99% of REE by leaving iron behind. FIG. 1c shows the acid consumption associated to each element of Montviel FC using the present method. As a result, the total acid consumption diminished significantly and 21% of the acid is used for REE extraction. Sr Al 3% Mn 1% 6% REE 5% AlSr 1% 2% Others 1% REE 7% Mn 8% Fe 44% Mg 17% Mg 10% Al 4% Mg 31% Fe 67% 3% a Ca 6% Sr 4% Ca 2% Ba Ca 19% Ba 9% REE 21% Mn 24% Ba 5% b c Figure 1 – Effect of pre-treatments on HCl consumption of REE extraction, a) Total HCl Consumption = 293 kg HCl (100%)/MT ore, No treatment, b) Total HCl Consumption = 191 kg HCl (100%)/MT ore, Total HCl Consumption = 63.2 kg HCl (100%)/MT ore KEYWORDS Rare earth elements, extractive metallurgy, Acid consumption, leaching, Iron, gangue minerals