Chapter 10 Notes The KINETIC MOLECULAR THEORY is based on
advertisement

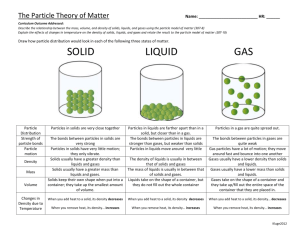
Chapter 10 Notes The KINETIC MOLECULAR THEORY is based on the idea that all particles of matter are in motion regardless to the state of matter (solid, liquid, or gas). It is very important to understand the physical properties and behavior of matter in the various states. In the case of gases, we must assume that are IDEAL or that a gas will meet all the 5 assumptions made concerning gases. 1. Gases consist of large numbers of tiny particles that are far apart from one another. 2. Collisions between two particles and particles and the container are ELASTIC, meaning there is no loss in kinetic energy. 3. They possess kinetic energy or they are in continuous, rapid, random motion. 4. There are no intermolecular forces between the particles. 5. The temperature of the gas is dependent on the average kinetic energy. Ideal gases do not actually exist because no gas will actually meet all 5 assumptions dealing with the average kinetic c theory. Gases do have intermolecular forces, although very weak, making all gases deviate from ideal gases. These gases are known as REAL GASES. However, the properties of gases can be easily explained using the kinetic molecular theory. Gases have no definite shape or volume. Gas particles move in all directions with very little attractive forces causing the gas to expand to fill larger volumes. Because the attractive forces are weak, the gas particles easily move past one another creating the ability to flow. Gases are concerned to be FLUIDS. The large distance between the particle of gas cause gases to have lower density than liquids or solids. The distance between the particles can be reduced when pressure is applied to the gas. This reduction in distance is known as compressibility. Gases will mix with each other without being stirred. This is known as DIFFUSION. Gases will also pass through small openings. This process is called EFFUSION. Liquids can be described as a form of matter that has a definite volume, but not a definite shape. According to the kinetic molecular theory, particles in a liquid are in constant motion: however, stronger intermolecular forces pull the particles closer together and lower the mobility of a liquid. Properties of liquids can also be explained using the kinetic molecular theory. The constant mobility, although slower than a gas, allows liquids to flow. Liquids are also considered to be fluids. The closer arrangement of particles makes the density of liquids 100 times higher than those of gases. The closer arrangement also limits the compressibility of liquids. The volume will decrease approximately 4% in a liquid. The constant motion allows for diffusion in liquids. Diffusion occurs at a much slower rate than a gas. The stronger intermolecular forces in a liquid create another property of liquids called SURFACE TENSION. This force pulls adjacent particles of a liquids surface together to decrease the surface area. Another property, capillary action, is closely related to surface tension. CAPILLARY ACTION is the attraction of a liquid surface to the surface of a solid. Solids are considered to have definite shape and definite volume. The kinetic molecular theory also explains the properties of a solid. Particles ion a solid are densely packed together. Motion is considered to be constant, but vibration around a fixed point. This differs from the random motion described in a gas or liquid. The intermolecular forces are considered to be strong. Solids are considered to have a more ordered structure than gases and liquids. Solids can be found in two forms. CRYSTALLINE SOLIDS contains crystals and are arranged in an orderly geometric pattern. AMORPHOUS SOLIDS are not considered to be crystalline and are arranged in a random pattern. Glass and Plastics are examples of amorphous solids. Solids will maintain a shape without the use of a container. The densely packed particles have very little space for compression to take place and are not considered to be compressible. Solids are not considered to be fluids because the particles are held to fixed positions by strong intermolecular forces. Most solids have higher density than gas or liquid. The short distance and strong intermolecular forces contribute to the higher density. Solids will diffusion, but the rate is millions of times slower than liquids. Matter can exist on earth in any of these states; gas, liquid, and solid. These states of matter are often called a PHASE, any part of a system that has uniform composition and properties. A change between these states of matter is known as a PHASE CHANGE. The various types of phase changes can be described below. The change from the solid phase to a liquid phase is known as MELTING. In turn, the phase change from a liquid to a solid is called FREEZING. The temperature at which this phase change occurs is known as the FREEZING POINT. The amount of energy involved in this phase change is known as the MOLAR ENTHAPY OF FUSION. It is the energy required to melt 1 mole of a solid. The change from a solid to a gas is called SUBLIMATION. Dry Ice (solid carbon dioxide) changing to carbon dioxide gas is an example. In turn, the change from a gas to a solid is called DEPOSITION. Water vapor changing to ice (snow) is an example of deposition. Liquid to a gas is called VAPORIZATION. EVAPORATION is similar to vaporization, but the particles escape the surface of a non-boiling liquid. Liquids that evaporate readily are considered to be VOLITE LIQUIDS. BOILING can be easily confused with these processes. Like evaporation, boiling is very similar to vaporization. Boiling can be defined as vaporization of a liquid within the body of the liquid. The temperature at which this process occurs is called the BOILING POINT. The MOLAR ENTHAPY OF VAPORIZATION is the amount of energy required to vaporize 1 mole of a liquid. The process of changing a gas to a liquid is called CONDENSATION. All phase changes can be graphically illustrated using a tool called a PHASE DIAGRAM. In a phase diagram, pressure and temperature and will show under which conditions needed for each phase to exist. A phase diagram is shown on page 347 of the textbook. The diagram is divided into three regions. Each region represents one of the three phases; gas, liquid, or solid. The curves on the diagram indicate the pressure and temperature required from a phase change to take place. There are three crucial points on a phase diagram. The TRIPLE POINT is the point where all three phase curves intersect. At this point, the needed pressure and temperature is indicated for all three phases to exist at the same time. The CRITICAL POINT indicates the critical temperature and critical pressure. Above this temperature, CRITICAL TEMPERATURE, no substance will exist in a liquid state. The pressure, CRITICAL PRESSURE, is the lowest pressure at which a liquid will exist.