Akshana Wettewa - Comparing Ionic and - tran-snc2dd
advertisement

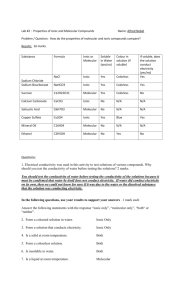
Lab Investigation Comparing Ionic and Molecular Substances Akshana Wettewa 9/30/2011 Comparing Ionic and Molecular Substances By: Akshana Wettewa Introduction: There are two kinds of ions; positive ions and negative ions. Positive ions are called Cations. Negative ions are called Anions. When an atom becomes an ion it wants to go back to neutral form so it makes bonds with other elements. One type of bond is an ionic bond. In ionic bonds it involves a transfer of electrons from a non-metal atom to a metal atom. Another type of bond is a covalent bond. A covalent bond is when a non-metal atom and another non-metal atom. An electrolyte is a substance that dissolves in water to produce ions. To find out if the compound is an electrolyte I tested it in water with a conductivity tester. If the LED light lights up then it is an electrolyte. Purpose: The purpose of the experiment is to examine the solubility in water and conductivity of ionic and molecular (covalent) bonds and also to find out what the unknown substances are. Hypothesis: If water is added, then the ionic mixtures will conduct electricity, since ionic compounds are made up of a metal atoms which is a good conductor of electricity. If water is added, then the molecular compounds will not conduct electricity, since molecular compounds are made up of non-metal atoms which are not good conductors of electricity. I think that the solubility for the ionic substances and molecular substances will change for each substance. Materials: Watch Glass Stir Rod Conductivity Tester Sample Beaker Wooden Splint Distilled Water Bottle Waste Beaker Ionic Substances Salt (NaCl) Potassium Chloride (KCL) Copper (II) Sulfate (CuSO4) Molecular Substances Simple Sugar (glucose) Starch Oil Unknown Substances A and B Procedure: Step 1: Using the bottle of distilled water add enough water droplets to the watch glass. Step 2: Take the wooden splint and place a small amount on to it, then drop it in the water. Step 3: Working with the glass stirring rod mix the water and the substance together. Give it some time to dissolve. Step 4: Put the conductivity tester into the solution to assess whether or not it conducts electricity. Record your observations. Step 5: Clear the waste out of the watch glass and drop it into a waste beaker. Make sure to rinse and dry the stir rod, watch glass and the conductivity tester before going to the next station. Independent Variable: Water Dependant Variables: Ionic substances, Molecular Substances The controlled variables are how much water and how much of the substances are put in. Observations: Table 1: Solubility and Conductivity of Ionic and Molecular Substances Substance Name Type of Substance (Ionic or Molecular) Solubility in Water (dissolved well/partially dissolved/did not dissolve) Observation when testing for Conductivity (light off/ light on steadily/light flashing) Conductivity (did not conduct/ conducted well/ conducted very well) Conducted very well Conducted very well Salt(NaCl) Ionic Dissolved well Light on steadily Potassium Chloride (KCL) Ionic Partially dissolved Light on steadily Copper (II) Sulfate (CuSO4) Ionic Dissolved well Light off Did not conduct Simple Sugar (glucose) Molecular Dissolved well Light off Did not conduct Starch Molecular Light on Conducted well Oil Unknown A Unknown B Molecular Molecular Ionic Partially dissolved Does not dissolve Dissolved well Dissolved well Light off Light off Light on steadily Did not conduct Did not conduct Conducted very well Analysis: Results: The salt and water mixture dissolved well and the light had stayed on steadily. This means that it conducted very well. Also when we put the conductivity tester into the salt and water mixture there appeared yellow and white fizz. The potassium chloride and water mixture partially dissolved and the light stayed on steadily. This means that the mixture conducted very well. The copper sulfate and water mixture dissolved well but the light was off. This means that it did not conduct electricity. The simple sugar (glucose) and water mixture dissolved well but the light was off. This means that the mixture did not conduct electricity. The starch and water mixture partially dissolved and the light was on but not very bright. This means that the mixture conducted electricity. Oil and water mixture did not dissolve and the light was off. This means it did not conduct electricity. The unknown A mixture dissolved well but the light was off. This means the unknown mixture did not conduct electricity. The unknown B mixture dissolved well and the light was on steadily. This means the unknown B mixture conducted very well. Error Analysis: In the experiment I think that there were some faults throughout the experiment. To be more accurate we should have measured the water and the amount of substance before mixing them. We may not have waited long enough for some of the mixtures to dissolve therefore changing our outcomes. Discussion: 1. The ionic substances for the most part dissolved fairly well. Salt and copper sulfate dissolved well and potassium chloride partially dissolved. As for the conductivity for the ionic substances it was different. Salt conducted very well because of the presence of ions. Also potassium chloride conducted very well. However, copper sulfate did not conduct any electricity. According to my results most ionic compounds dissolve well and conduct electricity. Of course there are exceptions as well in this case it was potassium chloride. 2. The molecular compounds had various stages of dissolving. Simple sugar (glucose) dissolved well were as starch only partially dissolved and oil did not dissolve at all. The molecular compounds for the most part did not conduct electricity. Simple sugar and oil did not conduct electricity. This is because simple sugar is an organic mixture therefore it does not conduct electricity. However starch conducted very well. According to my results most molecular substances do not conduct electricity but there always will be exceptions in this case it was starch. 3. I think that using these properties I could tell if the substance was molecular or ionic. If the unknown mixture conducted electricity well and dissolved well I would guess that it was an ionic compound. If the unknown mixture did not conduct electricity well I would guess that it was a molecular compound. 4. I think that unknown B mixture is potassium bromide because it dissolved well and conducted very well. Both of these properties are properties of ionic mixtures. Since potassium bromide is an ionic substance it has to be unknown B mixture. I think that unknown A mixture is table sugar (sucrose) because it dissolved well but did not conduct electricity. This was similar to simple sugar (glucose) which also had the same results. Both of these are properties of molecular substances. Since table sugar is a molecular compound it has to be unknown A mixture. Conclusion: In conclusion I found out that the electrolytes were salt, potassium chloride, potassium bromide and starch. The non electrolytes were copper sulfate, simple sugar (glucose), table sugar (sucrose) and oil. Most ionic compounds dissolve well and conduct electricity. Most molecular substances do not conduct electricity. In my hypothesis I was correct about ionic compounds being good conductors and molecular compounds being poor conductors. References McCurry John and Fay Robert C. Chemistry third edition. United States. "Electrolytes." Welcome to Waterloo Science | UW Faculty of Science. Web. 29 Sept. 2011. <http://www.science.uwaterloo.ca/~cchieh/cact/c120/electrolyte.html>.