IVIG - Yimg
advertisement

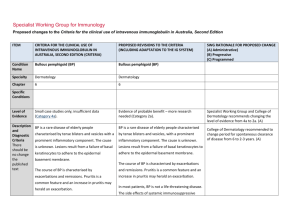
October 22, 2011 BlueCross BlueShield of Illinois RE: Patient: Provider: Service: IVIG Date of service: Prior authorization for IVIG (proposed start date 10/21/2011) Dear Medical Director: I am writing to initiate an appeal of BCBS-IL’s denial of coverage of Intravenous Immunoglobulin (IVIG) for treatment of recurrent pregnancy loss related to autoimmune disorders, as detailed in the predetermination letter dated October 11, 2011. BCBS-IL’s initial denial was based on the fact that there is insufficient evidence in support of the use of IVIG for these purposes in the peer-reviewed literature. However, the basis for this conclusion is both outdated and not applicable in this case. In addition, there is a large amount of medical literature supporting the use of IVIG in this case; this evidence comes from a wide variety of peer-reviewed journals. I have taken the time to compile a subset of this research literature, including references and summaries of the methods and results; you can find this information in the pages that follow. I have undergone three attempts at in vitro fertilization (IVF). I have been pregnant three times and five heartbeats have been recorded during those pregnancies. The first pregnancy was the result of a natural conception; since then I have been unable to conceive naturally and I have had one failed IVF cycle and two IVF cycles that resulted in pregnancy. Though no live birth followed the natural conception, infertility can be considered secondary, as the first pregnancy occurred naturally and within average latency. Missed miscarriages have followed each conception, all of which required dilation and curettage (in fact, one miscarriage required two D&Cs to remove all products of conception); testing results on the products of conception have indicated normal karyotypes, ruling out aneuploidy as the cause of embryonic/fetal demise. Moreover, male factor infertility has been ruled out, as have physical problems with my anatomy such as fibroids, uterine septum, and blocked fallopian tubes (documentation of these medical results available upon request). After the third pregnancy loss, I underwent a battery of immunologic tests. I tested positive for antinuclear antibodies (ANA) and my natural killer cell (NK) level and cytotoxicity were elevated. Increased killing activity is associated with implantation failure and pregnancy loss. Natural Killer cell activity or activation assay (NKa) measures the killing activity (cytotoxicity) within each cell, as well as the ability of IVIG to suppress the killing activity. Patients with high NK cell activity that suppress with IVIG in the NKa will respond very well to intravenous immunoglobulin (IVIG) therapy. In fact, the live birth rate with preconception IVIG is more than 80%, compared to 20% without treatment. My test results indicated that the NK cell in vitro response to immunoglobulin G was adequate. Additionally, I was found to have increased T Helper 1, inflammatory immune response. T Helper 1/T Helper 2 cytokine ratio reflects the ratio between two opposing T Helper immune responses. My elevated ratio reflects the dominance of TH-1 cells (represented by secreting TNF-alpha and INF-gamma), which are cytotoxic and pro-inflammatory; as against the TH-2 cells (represented by secreting IL-10) which are important for implantation and successful pregnancy. TNF-alpha is considered a major inflammatory mediator; it is a T-Helper 1 cytokine produced by activated macrophages, T cells, and other cells, and has many biological activities on the immune and reproductive system. My medical records and test results support Dr. Kwak Kim’s clinical judgment that future natural and IVF attempts at pregnancy will continue to fail unless I am able to be treated with IVIG, as does evidence found in the peer-reviewed literature. For these reasons, I ask that BCBSIL reverse its decision and permit me to undergo IVIG prior to my next and final attempt at IVF. In contrast to BCBC-IL’s claim that there is insufficient evidence to support the use of IVIG for these purposes, research has supported the effectiveness of this treatment in achieving and maintaining pregnancy and live birth. Historical and recent articles have shown that IVIG is effective, especially in cases of immunologic IVF failure like mine. Other treatments, such as prednisone therapy, are not appropriate in this case because of my predisposition to glucose sensitivity. Moreover, “Corticosteroid therapy has been shown to be ineffective for [spontaneous abortion] and this treatment is associated with numerous complications during pregnancy, especially preterm delivery” (Stricker, Steinleitner, Bookoff, & Weckstein, 2000). Thus, Dr. Kwak Kim feels that the presence of NK cells and ANA and cytokine ratios point strongly in favor of IVIG (see Doctor’s letter requesting pre-authorization for IVIG). Several authors, whose articles can be found below, explain how effective IVIG can be for a patient like me, who has failed IVF repeatedly and experienced recurrent pregnancy loss. Several other articles also indicate that a trial with IVIG is indicated at this point. Clark, Coulam, and Stricker (2006) found that “IVIG significantly increase[s] the probability of taking home one or more babies by patients undergoing IVF for infertility and/or early pregnancy loss.” They explain that, “increased numbers and/or activity of circulating bloodtype NK-related cells can translate into an unfavorable environment at the feto-maternal interface,” and add that “a common theme in patients with IVF failure/infertility and/or recurrent miscarriages has been increased NK activity, increased TH1/Th2 cytokine ratios, and presence of autoantibodies, and patients undergoing IVF have an increased miscarriage rate which . . . appears to be countered by IVIG.” van den Heuvel and colleagues report very convincing results (van den Heuvel, Peralta, Hatta, Han, & Clark, 2007). In their study “eight of thirty infertile women presented with high numbers of CD56+ CD3+ NKT cells, which declined after treatment with IVIG. The elevated NKT cell group with or without concomitant autoimmunity achieved a significantly higher successful pregnancy rate over the course of the study, as compared to women with average numbers of NKT cells and no evidence of autoimmunity (p = 0.018). Elevated NKT levels alone was an independent predictor of success on treatment (p = 0.003).” Stricker and colleagues (2000) findings also are impressive. Of the 47 women participating in this study, 36 received initial IVIG treatment, and 24 subsequently became pregnant. Of these women, 20 continued IVIG treatment through 26–30 weeks of gestation, and 19 (95%) had a successful term pregnancy. Four women discontinued IVIG therapy after 10–12 weeks of gestation, and 3 (75%) had a successful pregnancy outcome. Of the 11 women who refused IVIG therapy, 7 became pregnant, and all 7 miscarried. The difference in pregnancy success rate between the IVIG-treated and untreated groups was significant (p < .001). Authors also discussed problems with the design of other studies that that did not find such a beneficial effect. Those problems included poor patient selection that deliberately excluded older women and did not adequately screen for immunologic abnormalities. “The resultant comparison between younger women who have a high pregnancy success rate without any treatment significantly biased the outcome of these studies against IVIG therapy. Other problems include irrational or excessive IVIG regimens and inadequate patient screening for immunologic abnormalities.” Furthermore, Hutton and colleagues recently conducted a meta-analysis and found a significant increase in live births following IVIG use in women with secondary recurrent miscarriage (OR 2.71, 95% CI 1.09-6.73) in randomized controlled trials evaluating IVIG for treatment of spontaneous recurrent miscarriage (Hutton, Sharma, Fergusson, Tinmouth, Hebert, Jamieson, & Walker, 2007). For couples with immunological problems such as mine, the chances of losing a baby increase with each successive pregnancy. The next round of IVF would be our fourth and most likely final attempt. Our odds without IVIG are slim. Therefore, IVIG is the best remaining option for me and my husband, whose chance to carry a child to term is running out. The medical literature supports this use of IVIG as a safe and effective medication for treating immunological IVF failure. All alternatives present significant risks that are not present with IVIG. Anticoagulation therapy has been tried in isolation and is not helping. Corticosteroid therapy is ineffective and risky. Therefore, the best alternative is IVIG. Without effective treatment, my medical bills for another IVF attempt and likely D&C surgery will continue beyond the cost of IVIG. Thus, it is in both BCBS-IL’s interest and mine to try IVIG therapy. For all of these reasons, along with the additional evidence I summarize below, I urge you to reverse BCBS-IL’s noncoverage decision and give me a chance at the safe and effective treatment that my treating physician deems most appropriate. Of course, if you would like any additional information, please do not hesitate to contact me or Dr. Kwak Kim’s office. Thank you for considering this request, Selected References Birkenfeld A., Mukaida T., Minichiello L.., Jackson M., Kase N.G., & Yemini M. (1994). Incidence of autoimmune antibodies in failed embryo transfer cycles. American Journal of Reproductive Immunology, 31, 65-68. Summary: PROBLEM: The presence of antiphospholipid antibodies lupus anticoagulant (LAC), anticardiolipin antibody (ACA) as well as antinuclear antibody (ANA) has been associated with early spontaneous pregnancy loss and adverse pregnancy outcome. The purpose of this study was to investigate the possible role of autoimmune antibodies (LAC, ACA, and ANA) as a cause of implantation failure following embryo transfer (ET) after in vitro fertilization (IVF). METHOD: Three groups were studied: Group I, 56 patients who failed to conceive following ET; group II, 14 patients who have conceived following IVF-ET and delivered or are carrying an uncomplicated ongoing pregnancy; and group III, 69 patients who were new candidates for IVF-ET. RESULTS: Eighteen out of 56 (32.1%) of patients who failed to conceive following previous IVF-ET cycle (group I) tested positive for one or more of the autoimmune antibodies. None of the 14 patients of group II tested positive for autoimmune antibodies (P < .02). Seven out of the 69 patients (10%) of group III were found positive to one or more of the autoimmune factor. This rate is significantly lower than the rate of positive autoimmune antibodies detected in group I (P < .003). Fifteen patients of the 18 who tested positive for autoimmune antibodies and who had previously failed to conceive following ET underwent a subsequent IVF-ET cycle while being treated with prednisone and aspirin. Seven out of the 15 (46.6%) conceived and were able to sustain a clinical ongoing pregnancy. CONCLUSIONS: Patients receiving ET are carrying viable embryos within the intrauterine environment. Therefore, in this unique group of patients, failure to demonstrate a positive pregnancy test represents an implantation failure or a very early postimplantation loss. The results of this study suggest that periimplantation events may be affected by autoimmune antibodies. Very early miscarriage or implantation failure may be related to the same pathophysiological mechanism that causes recurrent miscarriages and is diagnosed incorrectly as infertility. Carp, H.J., Toder, V., Gazit, E., Ahiron, R., Torchinski, A., Mashiach, S., & Shoenfeld, Y. (2001). Further experience with intravenous immunoglobulin in women with recurrent miscarriage and a poor prognosis. American Journal of Reproductive Immunology, 46, 268-273. Summary: PROBLEM: Women with three or more unexplained miscarriages have a 60% chance of a subsequent live birth. Intravenous immunoglobulin (IVIG) has not been conclusively shown to improve this prognosis. This study assessed the effect of IVIG in patients expected to have a poor outcome if untreated, i.e. women with five or more abortions, who have aborted after paternal leukocyte immunization or who continue to abort despite expressing anti-paternal complement dependent antibody. METHODS: Seventy-six women received IVIG in a dose of 400 mg/kg body weight, in one day (total 30-45 g) in the follicular phase of a cycle in which pregnancy was planned. A booster dose was administered when pregnancy was diagnosed. Their results were compared to an untreated control group of 74 women. RESULTS: Thirty-five (49%) pregnancies in treated women have resulted in live births or passed their previous stages of abortion compared to 23 (31%) in control patients (P = 0.04). CONCLUSIONS: These figures indicate that IVIG may prevent further miscarriages in this poor prognosis population. These figures are especially significant considering the doubt concerning the efficacy of IVIG in patients with three miscarriages and therefore a relatively good prognosis. Christiansen, O.B., Pedersen, B., Rosgaard, A., & Husth, M. (2002). A randomized, doubleblind, placebo-controlled trial of intravenous immunoglobulin in the prevention of recurrent miscarriage:Evidence for a therapeutic effect in women with secondary recurrent miscarriage. Human Reproduction, 17, 809-816. Summary: BACKGROUND: Previous trials of intravenous immunoglobulin (IVIG) treatment of women with recurrent miscarriage (RM) have provided diverging results. This may be due to different inclusion criteria and suboptimal treatment protocols in some trials. METHODS: According to a computer-generated list, 58 women with at least four unexplained miscarriages were randomly assigned to receive infusions of high doses of IVIG or placebo starting as soon as the pregnancy test was positive. RESULTS: In the intention-to-treat analysis, a 45% live birth rate was found in both allocation groups. In patients with secondary RM, 50% in the treatment group and 23% in the placebo group had successful pregnancies (P = not significant). When data from the present and a previous placebo-controlled trial of the same treatment were combined, 15/26 (58%) of the patients with secondary RM in the treatment group versus 6/26 (24%) in the placebo group had successful outcomes (P < 0.02). Only 7% of the karyotyped abortuses were abnormal. CONCLUSIONS: IVIG may improve pregnancy outcome in patients with secondary RM. Clark DA, Coulam CB, Stricker RB. (2006) Is intravenous immunoglobulins (IVIG) efficacious in early pregnancy failure? A critical review and meta-analysis for patients who fail in vitro fertilization and embryo transfer (IVF). Journal of Assisted Reproduction & Genetics, 23, 1-13. Summary: Problem: Intravenous Immunoglobulins (IVIG) are widely used off label in the treatment of early reproductive failure. As IVIG is expensive, and may have side-effects, evidence of efficacy is needed. Previous results have suggested that the pre-conception treatment of primary recurrent abortion patients might be effective, but the data set has been too small for adequate statistical power. More recently it has been suggested that IVIG may improve the success rate of in vitro fertilization and embryo transfer (IVF) in patients with prior IVF failures, but clinical trials have given conflicting results that need explanation. Systematic reviews generating inconclusive results have focused on methodological rigor to the exclusion of biological plausibility. Methods: Review of current basic science of design, measurement, and evaluation of clinical trials and basic science mechanisms providing a rationale for treatment. Meta-analysis of published randomized controlled and cohort-controlled trials (updated with two unpublished data sets) evaluating IVIG treatment in IVF failure patients. Live birth rate was used as the most relevant endpoint. The ability of different sources of IVIG to suppress natural killer (NK) cell activity was determined using a standard 51Cr- release assay in vitro. Results and conclusions: Meta-analysis of three published randomized controlled trials (RCTs) of IVIG in IVF failure patients shows a significant increase in the live birth rate per woman (p = 0.012; Number Needed to Treat for 1 additional live birth, NNT = 6.0 women). Using live birth rate per embryo transferred, and adding data from two cohort-controlled trials to the meta-analysis further supports this conclusion (overall p = 0.000015, NNT = 3.7 women). Relevant variables appear to include properties and scheduling of the IVIG, and selection of patients with abnormal immune test results. Different IVIG preparations vary significantly in their ability to suppress NK activity in vitro. A rationale for use of IVIG is provided by a review of mechanisms of IVIG action and mechanisms underlying failure of chromosomally normal embryos. Coulam, C. B., & Goodman, C. (2000). Increased pregnancy rates after IVF/ET with intravenous immunoglobulin treatment in women with elevated circulating C56+ cells. Early Pregnancy, 4, 90-98. Summary: Intravenous (IV) immunoglobulin (Ig) has been previously shown to increase pregnancy rates after previously failed in vitro fertilization (IVF) embryo (ET) attempts in women who are efficient embryo producers (fertilize at least 50% of oocytes retrieved and generate at least 3 embryos/cycle). Women experiencing implantation failure have a higher frequency of elevated percentage of circulating CD56+ (natural killer) cells (>12%) than fertile women (3-12%). To evaluate the effects of IVIG on pregnancy rates in women with elevated percentage of circulating CD56+ cells, 32 women who had previously failed IVF/ET (>12 embryos transferred without pregnancy) were studied. Pregnancy and live birth rates with and without IVIG were compared in the same woman. All 32 women had previously failed to conceive after at least 12 ET, were efficient embryo producers and had persistently elevated plasma concentrations of CD56+ cells. Each woman received IVIG 500mg/kg prior to ET. If serum hCG concentrations were positive for pregnancy, IVIG was continued at 500mg/kg/mo until 28 weeks gestation. Pregnancy rates with and without IVIG were 56% and 9% (P<0.0001). The rate of live birth was 38% with IVIG and 0% without IVIG (P<0.0001). IVIG enhances pregnancy and live birth rates in women with elevated circulating CD56+ cells who have a history of implantation failure. Despite technologic advances leading to enhancement of fertilization rates after in vitro fertilization (IVF) (1, 2) implantation rates after embryo transfer (ET) have not increased significantly (3) over the last 20 years (4). Implantation rates after IVF/ET are influenced by the quality of the embryos and receptivity of the endometrium (3-9). Endometrial receptivity involves both hormonal (10-13) and immunologic (14-29) factors. Among the immunologic factors that play a crucial role in successful implantation are natural killer (NK) cells (14-18). NK cells present within the decidua that express CD56(but lack CD 16) have been associated with successful implantation (14-18). A deficiency of decidual CD56+ CD16- cells (18) and an increase in circulating CD56+ cells (25, 26) have been observed in women experiencing implantation failure. Women experiencing implantation failure after IVF and multiple ET have been successfully treated with intravenous (IV) immunoglobulin (Ig) (27). IVIG reduces activation of NK cells and NK killing activity both in vitro (29) and in vivo (30- 31). This reduction in activation of NK cells is essential for normal implantation to occur (14). To further define the role of IVIG for treatment of implantation failure, pregnancy and live birth rates were compared before and after IVIG treatment in women undergoing IVF/ET who had elevated levels of circulating CD56+ cells. Gleicher N., Liu H.L., Dudkievicz A., Rosenwaks Z., Kaberlien G., Pratt D., et al. (1994). Autoantibody profiles and immunoglobulin levels as predictors of in vitro fertilization success. American Journal of Obstetrics and Gynecology, 170, 1145-1149. Summary: STUDY DESIGN: This was a blinded study in which laboratory evaluations were performed on coded samples obtained from another institution. Codes were broken and data were analyzed after results of all laboratory tests had been reported out. One hundred five infertility patients who had undergone in vitro fertilization were randomly chosen. Among those, 46 were considered low responders (six or fewer oocytes were retrieved) and 59 as high responders (13 to 30 oocytes were retrieved). Total immunoglobulin G, M, and A levels and 15 autoantibody levels (6 antiphospholipids, 5 antihistones, and 4 antipolynucleotides) were determined separately for immunoglobulin G, immunoglobulin M, and immunoglobulin A isotypes. RESULTS: High and low responders demonstrated an unusual incidence of autoantibody (25% and 30%, respectively) and immunoglobulin (46% and 48%, respectively) abnormalities. They did not differ from each other, however, in either immunoglobulin or autoantibody parameters. Autoantibody and immunoglobulin abnormalities alone or in combination did not predict pregnancy success (24% vs 16%), incidence of chemical pregnancies (15% vs 24%), or clinical pregnancy loss (9% vs 11%) when such women were compared with those without either abnormality. However, the occurrence of hypergammaglobulinemias, in contrast to hypogammaglobulinemias, was associated with a significant decrease in the clinical pregnancy rate (6% vs 24%, p = 0.05). CONCLUSIONS: Neither autoantibody abnormalities nor total immunoglobulin abnormalities allow differentiation between high and low responders in in vitro fertilization cycles. The presence of autoantibody and total immunoglobulin abnormalities also does not predict low clinical pregnancy rates. Within a group of women with immunoglobulin abnormalities, those with hypergammaglobulinemias appear, however, at significant risk for low pregnancy rates with in vitro fertilization. This observation suggests that high total immunoglobulin levels may serve as a marker for an as yet to be determined immunologic factor that adversely affects the chance of conception. The evaluation of total immunoglobulin levels may be indicated as part of a routine infertility workup. Graphou, O., Chioti, A., Pantazi, A., Tsekoura, C., Kontopoulou, V., et al. (2003). Effect of intravenous immunoglobulin treatment on the Th1/Th2 balance in women with recurrent spontaneous abortions. American Journal of Reproductive Immunology, 49, 21-29. Summary: PROBLEM: The way by which intravenous immunoglobulin (IVIG) acts to prevent immunlogically mediated recurrent spontaneous abortions (RSA) has not been clarified. In the present study, a possible effect of IVIG on the T helper cell (Th1/Th2) balance was investigated in abortions of either alloimmune or autoimmune abnormalities. METHOD OF STUDY: The study included 21 women treated with IVIG before conception because of a history of RSA characterized by alloimmune abnormalities (n = 15) or associated with anti-phospholipid antibodies (APA) (n = 6). Peripheral blood samples, collected before and 5 days after the first IVIG infusion, were stimulated, and Th1 and Th2 cells were detected by flow-cytometric analysis using a combination of monoclonal antibodies against T-cell surface markers and intracellular interferon (IFN)-gamma and interleukin (IL)-4. The percentage of IFN-gamma-producing (Th1) and IL-4-producing (Th2) cells and the Th1/Th2 ratio were compared between pre- and post-infusion samples. RESULTS: A decrease of Th1 percentage in 66.6% of the cases and a concurrent Th2 percentage increase (47.61%) resulted in a decrease in the Th1/Th2 ratio in most of the cases (76.1%) (p < 0.01). Similar results were found in Group A (Th1/Th2 decreased in 60% of the cases, p < 0.05), while in Group B the effect of IVIG was not clear (Th1/Th2 increased in three and decreased in another three cases). CONCLUSION: Our finding suggests that IVIG administration in women with alloimmune RSA enhances Th2 polarization. This is not always the case with APA-associated abortions. Hutton, D., Sharma, R., Fergusson, D., Tinmouth, A., Hebert, P., Jamieson, J., & Walker, M. (2007). Use of intravenous immunoglobulin for treatment of recurrent miscarriage: A systematic review. BJOG: An International Journal of Obstetrics and Gynaecology, 114, 134-142. Summary: BACKGROUND: Intravenous immunoglobulin (IVIG) is a fractionated blood product whose off-label use for treating a variety of conditions, including spontaneous recurrent miscarriage, has continued to grow in recent years. Its high costs and short supply necessitate improved guidance on its appropriate applications. OBJECTIVE: We conducted a systematic review of randomised controlled trials evaluating IVIG for treatment of spontaneous recurrent miscarriage. SEARCH STRATEGY: A systematic search strategy was applied to Medline (1966 to June 2005) and the Cochrane Register of Controlled Trials (June 2005). SELECTION CRITERIA: We included all randomised controlled trials comparing all dosages of IVIG to placebo or an active control. DATA COLLECTION AND ANALYSIS: Two investigators independently extracted data using a standardised data collection form. Measures of effect were derived for each trial independently, and studies were pooled based on clinical and methodologic appropriateness. MAIN RESULTS: We identified eight trials involving 442 women that evaluated IVIG therapy used to treat recurrent miscarriage. Overall, IVIG did not significantly increase the odds ratio (OR) of live birth when compared with placebo for treatment of recurrent miscarriage (OR 1.28, 95% CI 0.78-2.10). There was, however, a significant increase in live births following IVIG use in women with secondary recurrent miscarriage (OR 2.71, 95% CI 1.09-6.73), while those with primary miscarriage did not experience the same benefit (OR 0.66, 95% CI 0.35-1.26). AUTHOR'S CONCLUSIONS: IVIG increased the rates of live birth in secondary recurrent miscarriage, but there was insufficient evidence for its use in primary recurrent miscarriage. Kwak Kim, J. Y., Gilman-Sachs, A., Beaman, K. D., & Beer, A. E. (1992). Reproductive outcome in women with recurrent spontaneous abortions of alloimmune and autoimmune causes: preconception versus postconception treatment. American Journal of Obstetrics and Gynecology, 166, 1787-1795. Summary: OBJECTIVES: The null hypothesis is that treatment of women with recurrent spontaneous abortions with anticoagulation and immunosuppression will not increase the reproductive outcome if it is started preconceptionally. STUDY DESIGN: Ninety-four women with recurrent spontaneous abortion with autoimmune abnormalities comprised the study group. Group I began autoimmune therapy 48 hours after ovulation: heparin 5000 U twice daily, aspirin 80 mg daily, and prednisone 5 mg twice daily, with an increase to 10 mg twice daily when pregnant. Group II started the same medication after a positive pregnancy test. Group III received no medication. Controls were 19 women with no autoimmune abnormalities. The frequency of reproductive outcome was subject to multiple comparison by the Duncan test. RESULTS: The percentages of live-born children in groups I, II, and III were 74%, 44%, and 11%, respectively. CONCLUSIONS: Preconception diagnostic work-up and treatment of autoimmune abnormalities in women with histories of recurrent spontaneous abortion is advocated. Kwak Kim, J. Y., Kwak, F. M., Gilman-Sachs, A., Beaman, K. D., Cho, D.D., & Beer, A.E. (2000). Immunoglobulin G infusion treatment for women with recurrent spontaneous abortions and elevated CD56+ natural killer cells. Early Pregnancy, 4, 154-164. Summary: We aimed to investigate the clinical effect of intravenous immunoglobulin G (IVIG) treatment in recurrent aborters with elevated peripheral blood CD56+ NK cell levels while on lymphocyte immunization, anticoagulation and prednisone treatment, with respect to subsequent live birth and reproductive outcome. Thirty-three women with recurrent abortions achieved alloimmune recognition after lymphocyte immunizations. All had autoimmune abnormalities and received preconception anticoagulation and prednisone treatment. At the time of positive pregnancy testing, 18 women with normal NK cell levels (<12%) and 6 with elevated NK cell levels (>12%) continued anticoagulation and prednisone treatment, and 9 with elevated NK cell level initiated additional IVIG treatment. The live birth rates of women with elevated NK cell level (>12%) who initiated post-conception IVIG treatment in addition to anticoagulation and prednisone (100.0%), women with normal NK cell levels (<12%) who continued anticoagulation and prednisone (83.3%) and women with elevated NK cell level (>12%) who continued anticoagulation and prednisone (33.3%) are significantly different (P=0.0065). Prevalence of intrauterine growth retardation and preterm delivery among 3 study groups were not different. In conclusion, post-conception IVIG treatment significantly improves reproductive outcome in women with elevated CD56+ NK cells with pregnancy who received preconception lymphocyte immunization, anticoagulation and prednisone treatment. Kwak Kim, J. Y., Quilty, E. A., Gilman-Sachs, A., Beaman, K. D., & Beer, A. E. (1995). Intravenous immunoglobulin infusion therapy in women with recurrent spontaneous abortions of immune etiologies. Journal of Reproductive Immunology, 28, 175-188. Summary: We have investigated clinical effectiveness of intravenous immunoglobulin G infusion (IVIG) on antiphospholipid antibody titers in five women with evidence of antiphospholipid antibody-associated recurrent spontaneous abortions and one with antinuclear antibody who became refractory to conventional autoimmune treatment during pregnancy and experienced pregnancy complications. Three women developed intrauterine growth retardation and three had complicated twin pregnancies with rising autoantibody titers. Antiphospholipid antibody and antinuclear antibody titers were tested pre and 2 weeks after each IVIG infusion. We report that: (i) IgG antiphospholipid antibody titers were significantly suppressed after each IVIG infusion (P < 0.05); (ii) IgM antiphospholipid antibody titers were also significantly suppressed after each IVIG infusion (P < 0.0001); (iii) decreased titers of autoantibodies paralleled increased levels of maternal IgG which lasted for at least 30 days; the autoantibodies showed a definite rise again prior to the next infusion; (iv) antinuclear antibody titers were effectively suppressed; and (v) rising autoantibody titers combined clinical manifestation of intrauterine growth retardation and women with complicated twin pregnancies. We conclude that IVIG infusion effectively suppresses IgM and IgG autoantibodies to phospholipids and antinuclear antibody in autoimmune women with a history of recurrent spontaneous abortions and refractory to conventional anticoagulation or immunosuppressive treatment. Morikawa, M., Yamada, H., Kato, E.H., Shimada, S., Kishi, T., Yamada, T., Kobashi, G., & Fujimoto, S. (2001). Massive intravenous immunoglobulin treatment in women with four or more recurrent spontaneous abortions of unexplained etiology: down-regulation of NK cell activity and subsets. American Journal of Reproductive Immunology, 46, 399-404. Summary: The aims of this study were to investigate the efficacy of massive intravenous immunoglobulin (MIVIG) treatment for women with recurrent spontaneous abortion (RSA) of unexplained etiology, and to investigate changes in peripheral natural killer (NK) cell activity and subsets. METHOD OF STUDY: MIVIG treatment was performed in 18 pregnancies from 15 women with 4 or more consecutive RSA of unexplained etiology. NK cell activity and subsets were assessed in 8 of the pregnancies. RESULTS: 14 pregnancies resulted in live births and 4 resulted in abortions with chromosome abnormality. The pre-infusion NK cell activity (mean + SD. 40.9 + 17.0%) at 4.4 +/- 0.5 weeks of gestation (GW) decreased to 15.0 +/- 7.90% at post-infusion status (5.4 +/- 0.5 GW). Pre-infusion percentages of CD56+ CD16- cells (3.5 +/- 2.1%) and CD56+ CD16cells (16.8 +/- 8.8%) decreased to 3.0 +/- 2.2% and 11.1 +/- 6.9%, respectively, after MIVIG treatment. CONCLUSIONS: MIVIG treatment was effective in all 14 pregnancies from RSA women of unexplained etiology, excluding 4 abortions with chromosome abnormality. Peripheral NK cell activity and subsets were suppressed by MIVIG treatment. Morikawa, M., Yamada, H., Kato, E. H., Shimada, S., Sakuragi, N., Fujimoto, S., & Minakami, H. (2003). Live birth rate varies with gestational history and etiology in women experiencing recurrent spontaneous abortion. European Journal of Obstetrics and Gynecology and Reproductive Biology,109, 21-26. Summary: OBJECTIVES: The aims of this study were to assess pregnancy outcome in relation to etiologic factors of recurrent spontaneous abortion (RSA). STUDY DESIGN: The pregnancies from consecutive 216 RSA women were assessed for live birth rates (LBR) according to etiology. The LBR in 110 pregnancies from RSA women with unexplained etiology was investigated according to various therapies. An attempt to karyotype the abortuses was made. RESULTS: Excluding pregnancies ending in abortion with abnormal karyotype, the LBR in primary recurrent spontaneous aborters (68.8%) who experienced three or more abortions was significantly lower than that in primary repeated aborters (82.4%) who experienced two abortions. The LBR ranged from 50 to 100% according to the etiology. In RSA women with unexplained etiology, the LBR in those undergoing massive intravenous immunoglobulin (MIVIG) therapy (100%) was significantly higher than those with low dose aspirin (57.1%) and luteal support therapy (67.3%). CONCLUSIONS: Excluding pregnancies ending in abortion with abnormal karyotype, we found that LBR varied with abortion history and etiologic factors of RSA. Scher, J. & Salazaar, C. (2000). Clinical experience with IVIG Rx in patients with prior failed IVF pregnancies: report of 30 consecutive patients. American Journal of Reproductive Immunology, 44, 121-124. Summary: PROBLEM: This study reviews one practitioner's experience with intravenous immunoglobulin (IVIG) therapy in the in-vitro fertilization (IVF) cycles of 30 patients with previous IVF failures. METHOD OF STUDY: Thirty patients had undergone 82 prior assisted reproductive technology (ART) cycles (mean 3.9 +/- 2 failed ART cycles, median 3.0, range 1-8) yielding one term birth, one loss at 22.5 weeks, and five chemical pregnancies. These patients underwent comprehensive clinical and laboratory evaluation, including immunologic workup, and were accepted for IVIG therapy in their next IVF cycle. RESULTS: A total of 40 cycles were treated. Twenty-four (60%) of the IVIGtreated IVF cycles showed a positive human chorionic gonadotropin test. Comparing the IVIG cycles to the untreated ART cycles, there were no differences in the number of embryos transferred, fertilized embryos, or eggs. Eighty-six percent of the cases with confirmed implantation delivered; there was one chemical pregnancy, one 20-week spontaneous fetal death, and one trisomy. Five (24%) of the 21 pregnant patients delivered at 30-36 weeks. The remaining 13 delivered at term. Only three (11%) had no positive immune test. CONCLUSION: In what may be a selected population of IVF patients (with high incidence of abnormal immune testing), early IVIG therapy may be associated with the improved success of IVF, and the high rate of live birth Sipak-Szmigiel, O., Ronin-Walknowska, E., & Miklaszwicz, A. (2003). Application of intravenous immunoglobulin therapy (IVIG) in pregnant patients with recurrent spontaneous abortions. Ginekologia Polska, 74, 35-355. Summary: OBJECTIVE: Clinical outcome of pregnancy in patients with recurrent pregnancy losses treated with IVIG during their current pregnancy. MATERIAL AND METHODS: The study group consisted of 10 pregnant women with 3-5 spontaneous abortions. Any genetic, anatomical or infectious abnormalities were excluded as well as antiphospholipid antibody syndrome and negative lymphocytotoxic test. The treatment consisted of passive immunotherapy by means of intravenous administration of immunoglobulin (IVIG) at a dose of 0.4 g/kg b.w. Therapy was commenced at week 5-6 of gestation, infusions were repeated every 3-4 weeks until 28th week of gestation in 5-, 3- and 1-day protocols. In 2 patients that therapy followed active immunotherapy with paternal lymphocytes. RESULTS: Eight (80%) patients bore healthy babies (3 preterm); in 2 cases embryos died at 6-7 week of gestation. CONCLUSIONS: The results confirm the efficacy of IVIG in pregnant patients with recurrent spontaneous abortions in whom likelihood of immunological pattern of pregnancy failures is high. Stricker, R.B., Steinleitner, A., Bookoff, C.N., Weckstein, L.N., & Winger, E.E. (2000). Successful treatment of immunologic abortion with low-dose intravenous immunoglobulin. Fertility and Sterility, 73, 536-540. Summary: OBJECTIVE: To evaluate the efficacy of low-dose intravenous immunoglobulin (IVIG) treatment in older women with immunologic abnormalities and recurrent spontaneous abortion (RSA), a condition referred to as immunologic abortion. DESIGN: Prospective clinical trial. SETTING: Outpatient referral practice. PATIENT(s): Fortyseven women were enrolled in the study. The mean age of the women was 37 years (range, 28-45 years), and the mean number of prior miscarriages was 3.7. Immunologic abnormalities included antiphospholipid antibodies (32%), antithyroid antibodies (53%), antinuclear antibodies (28%), antiovarian antibodies (2%), increased natural killer cells (40%), increased immunoglobulin (Ig)M level (28%), and increased CD4/CD8 T-cell ratio (15%). One patient had IgA deficiency, and three women had endometriosis. Thirtyone of the 47 patients (66%) had more than one immunologic abnormality. INTERVENTION(s): Treatment with IVIG at a dose of 0.2 g/kg within 2 weeks of attempted conception. Once conception was achieved, IVIG treatment was continued on a monthly basis at the same dose through 26-30 weeks of gestation. MAIN OUTCOME MEASURE(s): Successful pregnancy or recurrent abortion.Result(s): Of the 47 women, 36 received initial IVIG treatment, and 24 subsequently became pregnant. Of these women, 20 continued IVIG treatment through 26-30 weeks of gestation, and 19 (95%) had a successful term pregnancy. Four women discontinued IVIG therapy after 10-12 weeks of gestation, and 3 (75%) had a successful pregnancy outcome. Of the 11 women who refused IVIG therapy, 7 became pregnant, and all 7 miscarried. The difference in pregnancy success rate between the IVIG-treated and untreated groups was significant (P=.001). Three women had adverse reactions during the low-dose IVIG infusion, and these reactions resolved when the IVIG brand was changed. Fetal abnormalities were not observed. CONCLUSION(s): Low-dose IVIG therapy is beneficial for older women with immunologic abortion. Stricker, R.B., Steinleitner, A., & Winger, E. E. (2002). Intravenous immunoglobulin (IVIG) therapy for immunologic abortion. Clinical and Applied Immunology Reviews, 2, 187199. Summary: Recurrent pregnancy loss associated with immunologic abnormalities has been termed immunologic abortion. Immunologic abortion occurs primarily in women over the age of 30 years and may affect either natural or in-vitro fertilization (IVF)-induced pregnancy. In this article, we review the humoral and cellular immunologic abnormalities that have been associated with this form of recurrent abortion, and we discuss treatment options for women with this disorder. In particular, we have focused on intravenous immunoglobulin (IVIG) treatment for immunologic abortion. We analyzed 14 studies of IVIG therapy for recurrent loss of natural or IVF-induced pregnancies. Factors associated with successful use of IVIG were: (a) Older mean patient age; (b) inclusion of women with immunologic abnormalities; (c) initiation of IVIG therapy prior to conception; and (d) repeated administration of IVIG at physiologic intervals during pregnancy. When used according to these parameters, IVIG therapy is safe and effective for women with immunologic abortion. Stricker, R. B., & Winger, E. E. (2005). Update on treatment of immunologic abortion with low‐dose intravenous immunoglobulin. American Journal of Reproductive Immunology, 54, 390-396. Summary: PROBLEM: Recurrent spontaneous abortion associated with immunologic abnormalities has been termed immunologic abortion. Previously we showed that treatment with low-dose intravenous immunoglobulin (IVIG) appears to be beneficial for older women with immunologic abortion. We now report the results of IVIG treatment in a larger group of women with this disorder. METHOD OF STUDY: A total of 99 women were prospectively evaluated for immunologic abortion, which was defined as three or more miscarriages and the presence of specific immunologic abnormalities. Prior to the next conception, patients were treated with IVIG at a dose of 0.2 g/kg. Once conception was achieved, IVIG treatment was continued on a monthly basis through 26-30 weeks of pregnancy. RESULTS: The average age of the women was 37 years (range: 28-49), and the average number of miscarriages was 3.8 (range: 3-12). Of the 99 women, 72 received initial IVIG treatment, and 50 subsequently became pregnant. Of these women, 42 (84%) had a successful term pregnancy. Of the 27 women who refused IVIG therapy, 20 became pregnant and 18 (90%) miscarried. The difference in pregnancy success rate between the IVIG-treated and untreated groups was significant (P = 0.001). Four women had mild allergic reactions during IVIG infusion, and these reactions resolved when the IVIG brand was changed. Fetal abnormalities were not observed. CONCLUSION: We conclude that low-dose IVIG therapy is safe and effective for older women with immunologic abortion. van den Heuvel, M. J., Peralta, C. G., Hatta, K., Han, V. K., & Clark, D. A. (2007). Decline in number of elevated blood CD3+ CD56+ NKT cells in response to intravenous immunoglobulin treatment correlates with successful pregnancy. American Journal of Reproductive Immunology, 58, 447-459. Summary: Problem: Patients with elevated blood natural killer (NK) cells may be offered intravenous immunoglobulin (IVIG) treatment, but there is controversy about the utility of blood NK cell testing. Human CD56+ NK cells include several subpopulations that include the putatively cytotoxic CD56+CD16+ subset. In mouse models of pregnant failure, NKT cells appear to be important. However, a mouse model may only be pertinent to a subset of patients, as recurrent pregnancy failure is a heterogenous group. Method of Study: An ethics-approved observational study was done to observe the effect of treatment on total blood lymphoid cells, and subsets of CD56+ blood lymphocytes including CD56+ CD3+ NKT cells determined by flow cytometry, and to correlate with pregnancy outcome. Fifteen fertile women with a history of successful pregnancy and thirty-one women suffering from repeated implantation failure or recurrent spontaneous abortion provided serial blood samples during one menstrual cycle or prior to and during treatment. IVIG was administered to the latter group with or without heparin/aspirin. Result: Eight of thirty infertile women presented with high numbers of CD56+ CD3+ NKT cells, which declined after treatment with IVIG. The elevated NKT cell group with or without concomitant autoimmunity achieved a significantly higher successful pregnancy rate over the course of the study, as compared to women with average numbers of NKT cells and no evidence of autoimmunity (P = 0.018). Elevated NKT levels alone was an independent predictor of success on treatment (P = 0.003). Conclusion: Elevated NKT cells in recurrent pregnancy loss or implantation failure can be ameliorated with IVIG treatment, and result in successful pregnancy. Assay of NKT cell numbers may idengify patients who are more likely to benefit from IVIG therapy and merits further examination in randomized phase II studies.