Thermodynamics Homework: Refrigeration Cycles & Principles
advertisement

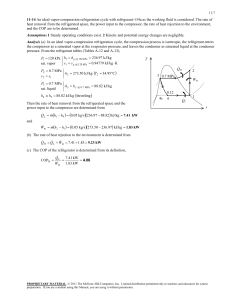
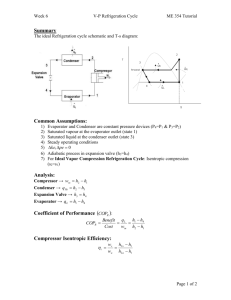
METU, ChE 204 May 13, 2014 Thermodynamics I, Section 06 Prof. Dr. Tülay Özbelge Asst. Emine Kayahan HOMEWORK-9 (Date Due: May 20, 2014) Solve these problems to study, but submit only Problems 3, 4, 6 and 7. 1) An ideal vapor-compression refrigeration cycle with a capacity of 3 tons has R-134a entering the compressor as saturated vapor at − 20C and exiting at 1MPa. a) Determine the coefficient of performance of the cycle, b) Determine the power input to the compressor. 2) A vapor-compression refrigeration cycle using R-134a has evaporator and condenser pressures of 201.7 kPa and 1MPa, respectively. The isentropic efficiency of the turbine is 0.85. Calculate the coefficient of performance. 3) A heat pump which operates on an ideal vapor-compression cycle with refrigerant-12 is used to heat water from 15C to 54C at a rate of 0.18 kg/s. The condenser and evaporator pressures are 1.4 and 0.32 MPa, respectively. Determine the power input to the heat pump. 4) Koretsky, 2nd ed, problem 3.62) Consider a refrigeration system based on an ideal vaporcompression cycle using R – 134a as the refrigerant. It operates between 0.7 MPa and 0.12 MPa with a flow rate of 0.5 mol/s. Calculate the following: a. the rate of heat removal from the refrigerant unit b. the power input needed to the compressor c. the COP The properties of R-134a can be found at http://webbook.nist.gov/chemistry/fluid/ or use the distributed data sheets. 5) An automobile air conditioner uses a vapor compression refrigeration cycle with environmentally friendly refrigerant HFC-134a as the working fluid. The following data are available for this cycle. Point 1 2 3 4 Fluid state Saturated liquid Vapor – liquid mixture Saturated vapor Superheated vapor Temperature 55⁰C 5⁰C a. Supply the missing temperatures, pressures, enthalpies and entropies for this cycle. b. Evaluate the coefficient of performance for the refrigeration cycle described in this problem. 6) By measuring the temperature of change and the specific volume change accompanying a small pressure change in a reversible adiabatic process, one can evaluate the derivative 𝜕𝑇 ( ) 𝜕𝑃 𝑠 and the adiabatic compressibility 𝐾𝑠 = − 1 𝜕𝑣 ( ) 𝑣 𝜕𝑃 𝑠 Develop an expression for (𝜕𝑇⁄𝜕𝑃 )𝑠 in terms of T, v, Cp, α, and KT and show that 𝐾𝑠 𝐶𝑣 = 𝐾𝑇 𝐶𝑝 7) Prove that the following statements are true. a. (𝜕𝐻⁄𝜕𝑣) 𝑇 is equal to zero if (𝜕𝐻⁄𝜕𝑃) 𝑇 is equal to zero. b. The derivative of (𝜕𝑆⁄𝜕𝑣)𝑃 for a fluid has the same sign as its coefficient of thermal expansion α and is inversely proportional to it.