Teacher: Chiquita Gaylor Week: 10/19
advertisement
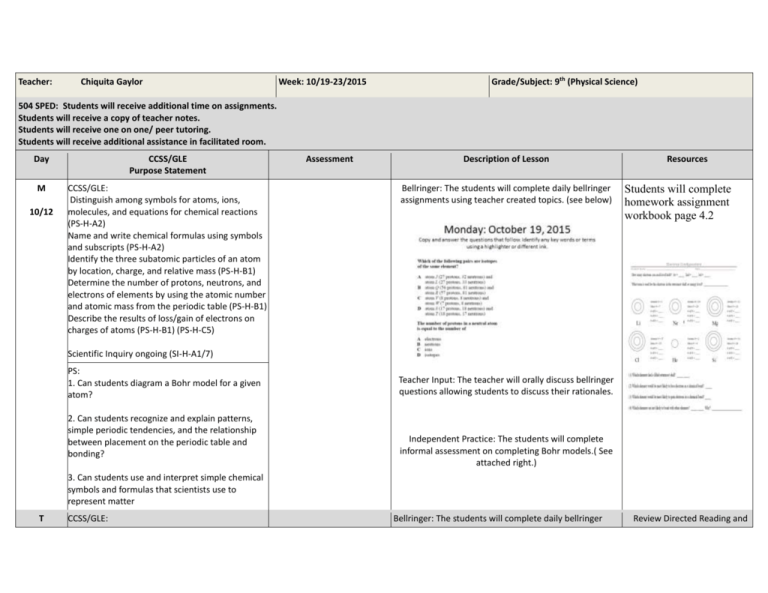
Teacher: Chiquita Gaylor Week: 10/19-23/2015 Grade/Subject: 9th (Physical Science) 504 SPED: Students will receive additional time on assignments. Students will receive a copy of teacher notes. Students will receive one on one/ peer tutoring. Students will receive additional assistance in facilitated room. Day CCSS/GLE Purpose Statement M CCSS/GLE: Distinguish among symbols for atoms, ions, molecules, and equations for chemical reactions (PS-H-A2) Name and write chemical formulas using symbols and subscripts (PS-H-A2) Identify the three subatomic particles of an atom by location, charge, and relative mass (PS-H-B1) Determine the number of protons, neutrons, and electrons of elements by using the atomic number and atomic mass from the periodic table (PS-H-B1) Describe the results of loss/gain of electrons on charges of atoms (PS-H-B1) (PS-H-C5) 10/12 Assessment Description of Lesson Bellringer: The students will complete daily bellringer assignments using teacher created topics. (see below) Resources Students will complete homework assignment workbook page 4.2 Scientific Inquiry ongoing (SI-H-A1/7) PS: 1. Can students diagram a Bohr model for a given atom? 2. Can students recognize and explain patterns, simple periodic tendencies, and the relationship between placement on the periodic table and bonding? Teacher Input: The teacher will orally discuss bellringer questions allowing students to discuss their rationales. Independent Practice: The students will complete informal assessment on completing Bohr models.( See attached right.) 3. Can students use and interpret simple chemical symbols and formulas that scientists use to represent matter T CCSS/GLE: Bellringer: The students will complete daily bellringer Review Directed Reading and 10/13 Distinguish among symbols for atoms, ions, molecules, and equations for chemical reactions (PS-H-A2) Name and write chemical formulas using symbols and subscripts (PS-H-A2) Identify the three subatomic particles of an atom by location, charge, and relative mass (PS-H-B1) Determine the number of protons, neutrons, and electrons of elements by using the atomic number and atomic mass from the periodic table (PS-H-B1) Describe the results of loss/gain of electrons on charges of atoms (PS-H-B1) (PS-H-C5) assignments using teacher created topics. (see below) Scientific Inquiry ongoing (SI-H-A1/7) PS: Teacher Input: The teacher will orally discuss bellringer questions allowing students to discuss their rationales. 1. Can students diagram a Bohr model for a given atom? Guided Practice: The students will exchange and grade homework assignment 4.2( workbook) 2. Can students recognize and explain patterns, simple periodic tendencies, and the relationship between placement on the periodic table and bonding? Independent Practice: Students will use small groups to complete study guide for the Introduction to Atoms test. Notes to prepare for upcoming student quiz. 3. Can students use and interpret simple chemical symbols and formulas that scientists use to represent matter W 10/15 CCSS/GLE: Distinguish among symbols for atoms, ions, molecules, and equations for chemical reactions (PS-H-A2) Name and write chemical formulas using symbols and subscripts (PS-H-A2) Identify the three subatomic particles of an atom by location, charge, and relative mass (PS-H-B1) Determine the number of protons, neutrons, and electrons of elements by using the atomic number and atomic mass from the periodic table (PS-H-B1) Bellringer: The students will complete daily bellringer assignments using teacher created topics. (see below) . Describe the results of loss/gain of electrons on charges of atoms (PS-H-B1) (PS-H-C5) Scientific Inquiry ongoing (SI-H-A1/7) PS: 1. Can students diagram a Bohr model for a given atom? 2. Can students recognize and explain patterns, simple periodic tendencies, and the relationship between placement on the periodic table and bonding? 3. Can students use and interpret simple chemical symbols and formulas that scientists use to represent matter Teacher Input: The teacher will orally discuss bellringer questions allowing students to discuss their rationales. Students will be instructed in how to complete a content article critique. See initial article attached. . Independent Practice: Students will demonstrate knowledge of atoms and their components by completing a teacher created exam with minimum of 68%. Th 10/15 CCSS/GLE: Differentiate between Bellringer: The students will complete daily bellringer Distinguish among symbols for atoms, ions, observations, inferences and assignments using teacher created topics. (see below) molecules, and equations for chemical reactions predictions (Worksheet) (PS-H-A2) Use experiment scenarios to Name and write chemical formulas using symbols identify the steps in the and subscripts (PS-H-A2) scientific method. ( BeriBeri) Identify the three subatomic particles of an atom by location, charge, and relative mass (PS-H-B1) Determine the number of protons, neutrons, and electrons of elements by using the atomic number and atomic mass from the periodic table (PS-H-B1) Homework: The students will complete workbook page 37-38 (4.2) The Structure of an Atom Describe the results of loss/gain of electrons on charges of atoms (PS-H-B1) (PS-H-C5) Scientific Inquiry ongoing (SI-H-A1/7) PS: 1. Can students diagram a Bohr model for a given atom? 2. Can students recognize and explain patterns, simple periodic tendencies, and the relationship between placement on the periodic table and bonding? 3. Can students use and interpret simple chemical symbols and formulas that scientists use to represent matter Teacher Input: The teacher will orally discuss bellringer questions allowing students to discuss their rationales. Guided Practice: Students will complete 10 minute ACT prep activity and orally discuss outcomes and results. The teacher will use powerpoint and lecture to initiate the periodic table. F 10/9 CCSS/GLE: Distinguish among symbols for atoms, ions, molecules, and equations for chemical reactions (PS-H-A2) Name and write chemical formulas using symbols and subscripts (PS-H-A2) Identify the three subatomic particles of an atom by location, charge, and relative mass (PS-H-B1) Determine the number of protons, neutrons, and electrons of elements by using the atomic number and atomic mass from the periodic table (PS-H-B1) Describe the results of loss/gain of electrons on charges of atoms (PS-H-B1) (PS-H-C5) Bellringer: The students will complete daily bellringer assignments using teacher created topics. (see below) Scientific Inquiry ongoing (SI-H-A1/7) PS: 1. Can students diagram a Bohr model for a given atom? 2. Can students recognize and explain patterns, simple periodic tendencies, and the relationship between placement on the periodic table and bonding? 3. Can students use and interpret simple chemical symbols and formulas that scientists use to represent matter Teacher Input: The teacher will continue discussion on properties of matter using power-points, handouts, and sample text resources. Independent Practice: The teacher will use powerpoint and lecture to initiate the periodic table.