INSTRUCTIONS TO AUTHORS FOR THE PREPARATION
advertisement
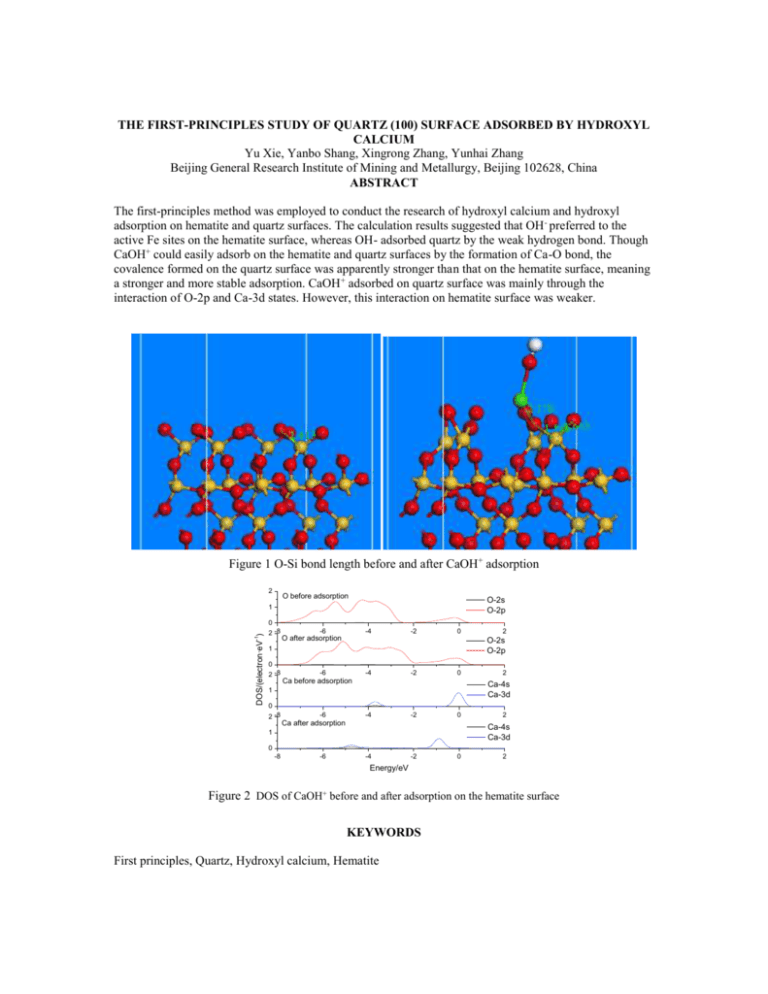
THE FIRST-PRINCIPLES STUDY OF QUARTZ (100) SURFACE ADSORBED BY HYDROXYL CALCIUM Yu Xie, Yanbo Shang, Xingrong Zhang, Yunhai Zhang Beijing General Research Institute of Mining and Metallurgy, Beijing 102628, China ABSTRACT The first-principles method was employed to conduct the research of hydroxyl calcium and hydroxyl adsorption on hematite and quartz surfaces. The calculation results suggested that OH - preferred to the active Fe sites on the hematite surface, whereas OH- adsorbed quartz by the weak hydrogen bond. Though CaOH+ could easily adsorb on the hematite and quartz surfaces by the formation of Ca-O bond, the covalence formed on the quartz surface was apparently stronger than that on the hematite surface, meaning a stronger and more stable adsorption. CaOH+ adsorbed on quartz surface was mainly through the interaction of O-2p and Ca-3d states. However, this interaction on hematite surface was weaker. Figure 1 O-Si bond length before and after CaOH+ adsorption 2 O before adsorption O-2s O-2p 1 DOS/(electron·eV-1) 0 2 -8 -6 -4 -2 0 O after adsorption 2 O-2s O-2p 1 0 2 -8 -6 -4 -2 0 Ca before adsorption 2 Ca-4s Ca-3d 1 0 2 -8 -6 -4 -2 0 Ca after adsorption 2 Ca-4s Ca-3d 1 0 -8 -6 -4 -2 0 2 Energy/eV Figure 2 DOS of CaOH+ before and after adsorption on the hematite surface KEYWORDS First principles, Quartz, Hydroxyl calcium, Hematite