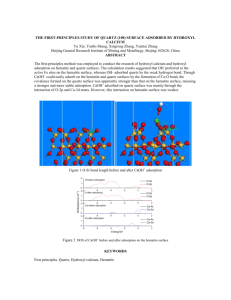
INSTRUCTIONS TO AUTHORS FOR THE PREPARATION
advertisement
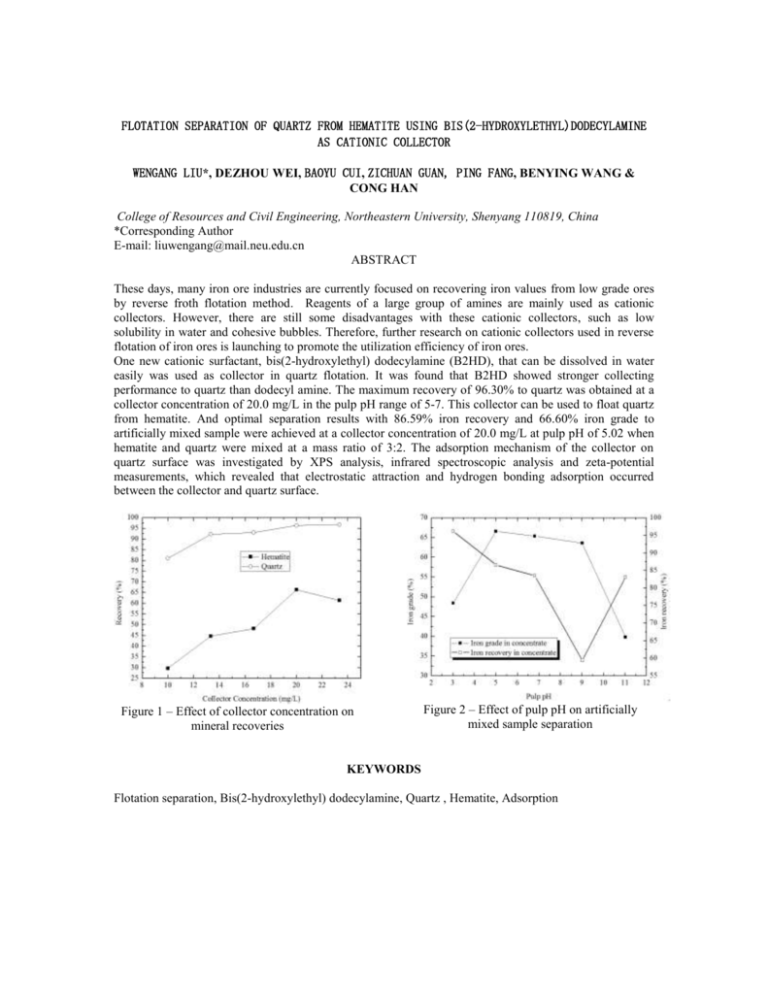
FLOTATION SEPARATION OF QUARTZ FROM HEMATITE USING BIS(2-HYDROXYLETHYL)DODECYLAMINE AS CATIONIC COLLECTOR WENGANG LIU*, DEZHOU WEI, BAOYU CUI, ZICHUAN GUAN, PING FANG, BENYING WANG & CONG HAN College of Resources and Civil Engineering, Northeastern University, Shenyang 110819, China *Corresponding Author E-mail: liuwengang@mail.neu.edu.cn ABSTRACT These days, many iron ore industries are currently focused on recovering iron values from low grade ores by reverse froth flotation method. Reagents of a large group of amines are mainly used as cationic collectors. However, there are still some disadvantages with these cationic collectors, such as low solubility in water and cohesive bubbles. Therefore, further research on cationic collectors used in reverse flotation of iron ores is launching to promote the utilization efficiency of iron ores. One new cationic surfactant, bis(2-hydroxylethyl) dodecylamine (B2HD), that can be dissolved in water easily was used as collector in quartz flotation. It was found that B2HD showed stronger collecting performance to quartz than dodecyl amine. The maximum recovery of 96.30% to quartz was obtained at a collector concentration of 20.0 mg/L in the pulp pH range of 5-7. This collector can be used to float quartz from hematite. And optimal separation results with 86.59% iron recovery and 66.60% iron grade to artificially mixed sample were achieved at a collector concentration of 20.0 mg/L at pulp pH of 5.02 when hematite and quartz were mixed at a mass ratio of 3:2. The adsorption mechanism of the collector on quartz surface was investigated by XPS analysis, infrared spectroscopic analysis and zeta-potential measurements, which revealed that electrostatic attraction and hydrogen bonding adsorption occurred between the collector and quartz surface. Figure 1 – Effect of collector concentration on mineral recoveries Figure 2 – Effect of pulp pH on artificially mixed sample separation KEYWORDS Flotation separation, Bis(2-hydroxylethyl) dodecylamine, Quartz , Hematite, Adsorption