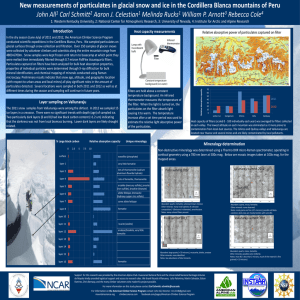
INSTRUCTIONS TO AUTHORS FOR THE PREPARATION
advertisement

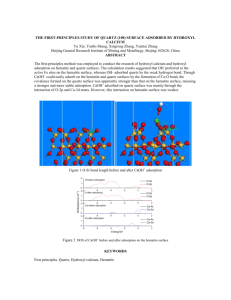
DENSITY FUNCTIONAL THEORY SIMULATION STUDY ON THE HYDROXYLATED HEMATITE SURFACES: UNDERSTANDING ITS REACTIVITY Vinay Jaina,*, Beena Raia, Pradipa and Umesh V. Waghmareb a Tata Consultancy Services Ltd., Tata Research Development and Design Centre (TRDDC), 54B, Hadapsar Industrial Estate, Pune – 411013, Maharashtra, India. b Jawaharlal Nehru Centre for Advanced Scientific Research, Jakkur, Bangalore-560 064, India *Corresponding Author: Phone: +91-020-66086305, Email: vinay2.j@tcs.com ABSTRACT The hematite surface plays a vital role in several processes of industrial and technological importance, such as, in beneficiation of iron ores, catalysis, and geochemical processes. Therefore, it is imperative to have a clear description of the structure and composition of this surface, which forms the underlying basis for such a wide variety of complex phenomena. Particularly, from the perspective of beneficiation of Indian alumina-rich iron ore slimes and fines, a clear understanding of the surface structure and composition of hematite surface and its difference from the alumina surface will facilitate identification and/or design of selective reagents which can be used to separate the two minerals. In this work, the hydroxylated hematite (0001) surface was modeled using density functional theory (DFT). The computed planar relaxations are consistent with X-Ray crystal truncation rod diffraction results reported in the literature. It is demonstrated that only one type of surface, that is, the (OH)3-Fe-H3O3-R is stable under aqueous conditions, as the relaxations for the second type of termination: (OH) 3-Fe-Fe-R do not match quantitatively with experiments suggesting that its coexistence with the (OH) 3-Fe-H3O3-R surface is unlikely. A detailed analysis of this surface shows that two of the three (OH) groups are not connected to the Fe surface atom. Instead, they form H2O molecules by combining with the H-atoms on the O-layer beneath the Feterminated surface leaving the Fe surface-atom bonded to only one OH-group. This under-coordination of Fe-atoms on the surface explains its higher reactivity as compared to hydroxylated alumina. (a) (b) Figure 1 – DFT Computed (a) (HO)3-Fe-Fe-R and (b) (HO)3-Fe-H3O3-R terminations in hydroxylated hematite (0001). Red, indigo and blue spheres indicate O, Fe, and H atoms respectively. Dotted lines indicate hydrogen bonds. KEYWORDS Hydroxylation, Hematite, Density functional theory; iron ore slimes; topography, surface structure