Lew Dot Structures
advertisement

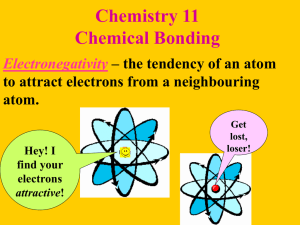
A visual representation of covalent compounds 1. Octet Rule ◦ Covalent compounds share electrons so that each atom has an octet of electrons (8) in its highest energy level. Exceptions: Hydrogen has a full valence shell at 2 electrons Boron has a full valence shell at 6 electrons instead of 8 2. Single Bonds ◦ 1 electron pair is shared (a total of 2 electrons) 3. Double Bonds ◦ 2 electron pairs are shared (a total of 4 electrons) 4. Triple Bonds ◦ 3 electron pairs are shared (a total of 6 electrons) Electron-dot notation can also be used to represent the structure of molecules, called Lewis Structures. The pair of dots between the two symbols represents the shared electron pair of the hydrogen-hydrogen covalent bond. In addition, each fluorine atom is surrounded by three pairs of electrons that are not shared in bonds. An unshared pair, also called a lone pair, is a pair of electrons that is not involved in bonding and that belongs exclusively to one atom. Step 1 Get a covalent formula and draw the electron-dot notation for each atom in the formula. Step 2 From the electron-dot notation, determine how many bonds each atom needs to get an octet. Step 3 Determine the central atom (Skip this step if there are only 2 atoms): ◦ Carbon is always the central atom ◦ If there is more than 1 carbon they make a chain in the center. -C-C-C- ◦ Hydrogen & group 17 elements are never in the center. ◦ The least electronegative is in the center. Step 4 Spread the remaining elements around the central atom & bond each to the center